Abstract
The prevailing model of osteology is that bones constantly undergo a remodeling process, and that the differentiation and functions of osteoblasts are partially regulated by leptin through different central hypothalamic pathways. The finding that bone remodeling is regulated by leptin suggested possible endocrinal effects of bones on energy metabolism. Recently, a reciprocal relationship between bones and energy metabolism was determined whereby leptin influences osteoblast functions and, in turn, the osteoblast-derived protein osteocalcin influences energy metabolism. The metabolic effects of bones are caused by the release of osteocalcin into the circulation in an uncarboxylated form due to incomplete γ-carboxylation. In this regard, the Esp gene encoding osteotesticular protein tyrosine phosphatase is particularly interesting because it may regulate γ-carboxylation of osteocalcin. Novel metabolic roles of osteocalcin have been identified, including increased insulin secretion and sensitivity, increased energy expenditure, fat mass reduction, and mitochondrial proliferation and functional enhancement. To date, only a positive correlation between osteocalcin and energy metabolism in humans has been detected, leaving causal effects unresolved. Further research topics include: identification of the osteocalcin receptor; the nature of osteocalcin regulation in other pathways regulating metabolism; crosstalk between nutrition, osteocalcin, and energy metabolism; and potential applications in the treatment of metabolic diseases.
In the last decade, clinical observations and controlled studies have shown that leptin produced by fat tissue regulates bone metabolism through a central pathway consisting of the hypothalamus, sympathetic nervous system, and multiple intermediary steps. This relationship between fat and bone, initially thought to be unilateral, is now considered to be bilateral or reciprocal after the discovery of the role of bone in glucose and fat metabolism. This newly identified feedback loop between bone and energy metabolism is mediated by osteocalcin (OC), an osteoblast-produced protein, and has advanced academic progress in osteology and endocrinology. Furthermore, studies of OC have provided the fundamental basis of therapeutic strategies for metabolic disorders. In this review, we discuss the feedback loop between bones and fat, and new discoveries related to novel metabolic roles of OC.
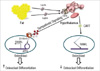
The prevailing model in skeletal biology is that bones constantly undergo the physiological process of bone remodeling, which comprises two phases: bone resorption by osteoclasts followed by bone formation by osteoblasts (1). Imbalance between the two phases whereby osteoclast activity surpasses osteoblast activity leads to decreased bone mineral density and, in turn, an increased risk of osteoporosis. A number of published studies have demonstrated that differentiation and functions of these bone-specific cells are regulated by leptin, an adipocyte-derived hormone that regulates energy intake and expenditure. Numerous other studies have examined the relationship between serum leptin levels and bone anabolism, and have proposed a bimodal response in which moderate increases in leptin stimulate bone formation whereas higher levels actually inhibit it (2-4). This bimodal response is further complicated by the finding that adipocytes in both the periphery and in the bone marrow secrete leptin, which may induce apoptosis of bone marrow stromal cells and favors bone resorption at high local concentrations (5, 6). It has been proposed that leptin exerts osteogenic functions through a peripheral pathway that directly targets a leptin-specific receptor on osteoblasts, but the results of relevant studies were inconsistent (7, 8). Other researchers have consistently shown that leptin regulates bone formation through a central pathway comprising the hypothalamus and central nervous system (9-15). Leptin is known to initiate intracellular signals within the hypothalamus through its binding to Ob-Rb, the leptin receptor isoform present in hypothalamic nuclei (16). At least two different central hypothalamic pathways, through which leptin influences bone formation in an antagonistic manner, have been identified (Fig. 1) (9-15). The first, anti-osteogenic, influence of leptin involves up-regulation of receptor activators of NF-kappa B ligand (RANKL), an osteoclast differentiation factor, through sympathetic signaling via β2-adrenergic receptors, the only adrenergic receptor present on osteoblasts (12, 15). Sympathetic signaling on β2-adrenergic receptors induces phosphorylation of activating transcription factor 4 (ATF4), a cell-specific cAMP response element binding (CREB)-related transcription factor that is essential for osteoblast differentiation and function (16). The second, more recently defined, osteogenic influence of leptin involves modulation of cocaine and amphetamine-regulated transcript (CART) (12), a hypothalamic neuropeptide encoded by the CARTPT gene whose expression is increased by leptin (17). Down-regulation of RANKL expression by CART is unfavorable to bone resorption and inhibits osteoclast differentiation (12, 13).
The concept of hormonal regulation in which hormones are regulated by a feedback loop, the facts that osteoblasts and adipocytes develop from a common precursor cell, the mesenchymal stem cell (15), and that bone remodeling is regulated by an adipocyte-derived hormone, leptin, have all aroused interest among integrative physiologists regarding the possible endocrinal effects of bone on energy metabolism (18). Moreover, an unexpected result of investigations on osteocalcin, a non-collagenous protein that is produced by osteoblasts and is involved in mineralization and calcium homeostasis, showed that osteocalcin gene knockout (OC-/-) animal models had an abnormal amount of visceral fat, thus providing the first evidence of a feedback loop between the skeleton and energy metabolism (19). This has led some researchers to conduct investigations to determine whether bone should be considered a ductless endocrine organ in addition to its classic functions of providing a framework to support the body and calcium homeostasis.
In 2007, a revolutionary reciprocal relationship between bones of the skeleton and energy metabolism was proposed, whereby leptin influences osteoblast functions and bone remodeling and OC in turn influences energy metabolism (20). The fact that only a limited number of osteoblast-specific genes encode functional molecules made it easy to examine whether transgenic constructs or deletion of these genes resulted in alterations in energy metabolism. Among these genes, the Esp gene encoding protein tyrosine phosphatase (OST-PTP) was of particular interest. Previous studies showed that the Esp gene is up-regulated following differentiation and matrix formation of primary osteoblasts and down-regulated in mineralizing osteoblasts (21-23). Deletion of the Esp gene in mice (Esp-/-) resulted in a high neonatal death rate from severe hypoglycemia associated with hyperinsulinemia and enhanced insulin sensitivity mediated by increased adiponectin production from adipocytes (20). This finding fostered the new hypothesis that osteoblast-specific OST-PTP may play a critical role in regulating glucose homeostasis.
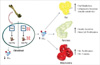
What makes the Esp gene and OST-PTP particularly interesting with respect to the relationship between bones and energy metabolism? In order to answer this question, a thorough understanding of the biophysical and biochemical properties of OC is required. OC is a vitamin K-dependent protein present in bones, and contains three glutamic acid residues that are posttranslationally modified by γ-glutamyl carboxylase (24, 25). This γ-carboxylation is essential for the protein to have a high affinity for mineral ions and enables the OC protein to attract calcium ions and incorporate these into hydroxyapatite crystals, which make up approximately 70% of bones (24). However, not all of the three glutamic acid residues in OC are fully carboxylated and incorporated into hydroxyapatite crystals, and the degree of carboxylation may vary. Uncarboxylated or undercarboxylated glutamic acid residues make OC susceptible to release from osteoblasts into the circulation (26). Hinoi et al. (27) demonstrated that leptin induces up-regulation of Esp expression on osteoblasts through sympathetic signaling without affecting overall production of OC, but causing a decrease in the serum concentration of uncarboxylated OC (OCuc). This study confirmed the report by Lee et al. (20) demonstrating increased concentrations of circulating OCuc in Esp-/- mouse models, besides providing further evidence that fat regulates bone metabolism. Although the exact mechanism is unclear, it is assumed that inhibiting OST-PTP expression negatively influences γ-car-boxylation of OC, thus promoting the release of OC into the circulation in the form of OCuc. Serum OCuc has been negatively associated with bone mineral density at measured bone sites (28-33). Surprisingly, serum OCuc appears to be sufficiently physiologically active to regulate energy metabolism, allowing bone to function as an endocrine organ (Fig. 2) (20, 34). Esp-/- mice representing OCuc-gain-of-function would be appropriate to determine the beneficial consequences of a high serum concentration of OCuc ([OCuc]) on energy metabolism.
Lee et al. (20) compared the effects of different [OCuc] on energy metabolism in genetically modified animal models (OC-/- and Esp-/-) and found that Esp-/- animals with significantly higher [OCuc] appeared to be protected from obesity, as evidenced by higher energy expenditure and reduced body weight and fat accumulation after being fed a high fat diet compared with wild-type (WT) and OC-/- models. Esp-/- models also exhibited lower serum levels of triglyceride and free fatty acids, suggesting that OCuc plays a role in lipid metabolism. OCuc also improved the insulin secretion capacity of the pancreas as measured by glucose-stimulated insulin secretion test and insulin tolerance test. This was evidenced by increased expression of MKI-67 and cyclin D, markers for cellular proliferation, in samples of pancreatic cells and a subsequent increase in pancreatic β-cell mass and area, and by increased secretion of adiponectin, an insulin-sensitizing adipocytokine, in Esp-/- animal models. OCuc also appeared to improve glucose tolerance determined by a glucose tolerance test and glucose infusion rate test. It is well known that reduction of mitochondrial content results in decreased mitochondrial function and leads to impaired energy metabolism and insulin resistance (35-37). Molecular and morphological analyses of the gastrocnemius muscle of Esp-/- animal models by Lee et al. (20) revealed an almost threefold increase in mitochondrial area and concurrent increase in the level of proteins associated with mitochondrial biogenesis including Mcad, PPAR-γ, acyl-CoA, UCP2, PGC1α, and NRF1 (38), suggesting that mitochondrial content and function were enhanced by OCuc. In contrast, OC-/- models displayed opposite characteristics of impaired lipid and glucose metabolism, and decreased insulin secretion and sensitivity (20). Thus, the revolutionary finding that OCuc positively regulates energy metabolism through increased fat metabolism, energy expenditure, insulin secretion capacity of pancreas, and release of adiponectin from adipocytes, and by inducing mitochondrial proliferation and function, established a complex crosstalk between bones and adipocytes in energy metabolism.
Evidence that OCuc enhances energy metabolism was confirmed not only in OC-/- mice exhibiting obesity, hyperglycemia, glucose intolerance, and insulin resistance, but also in WT mice (34). Ferron et al. (34) showed that OCuc had similar effects on energy metabolism in WT mice fed either a normal diet or a diet favoring obesity and type 2 diabetes after administration of OCuc. This study is especially notable as it proposes a pharmacological therapeutic potential of OCuc for obesity and type 2 diabetes. OCuc may also provide a novel treatment for sarcopenia, the loss of muscle mass and function with aging, when we consider the facts that activation of apoptosis initiated by mitochondrial dysfunction contributes to sarcopenia, and that OCuc induces mitochondrial proliferation and functional enhancement by increasing expression of proteins associated with mitochondrial biogenesis and functions (38, 39).
Although an association between OC and energy metabolism has been demonstrated in mice, it is necessary to address the question of whether these metabolic functions of OC also exist in humans. Prior to the identification of the novel metabolic roles of OCuc in 2007, human studies performed over several decades reported markers of low bone turnover, including OC, in diabetic patients (40-42). Although OC has been extensively investigated in bone biology and related fields, it was not until very recently that OCuc was investigated in humans with respect to its metabolic functions. Im et al. (43) reported a significant reduction in [OC] among type 2 diabetic patients compared with normal glucose and impaired fasting glucose groups. These authors also showed significantly decreased fasting glucose and HbA1c levels in the highest quartile group for [OC] compared with the lowest quartile group in a study involving 339 Korean postmenopausal women (43). This inverse relationship was confirmed in studies on 1010 elderly Swedish men by Kindblom et al. (44) and studies on 380 elderly males and females by Pittas et al. (23). Human studies commonly reported an inverse relation between [OC] and fat mass and plasma glucose level; multivariate analyses by Im et al. (43) and Kindblom et al. (44) showed that [OC] is an independent negative predictor of plasma glucose, and Pittas provided the first demonstration that [OC] is inversely associated with the homeostasis model assessment for insulin resistance (HOMA-IR) in humans (23). Although [OCuc] was not measured, such observations suggest a potential metabolic role of OCuc in glycemia and adiposity in humans similar to that in the animal studies. It, however, would be premature to conclude a causal effect of OCuc on the regulation of energy metabolism in humans as the above studies investigated only correlations. Furthermore, these studies are limited by the fact that [OC] has been reported to vary with multiple factors including nutritional status, age, gender, smoking status, physical fitness levels, and season of the study (45, 46).
As mentioned above, [OC] varies with multiple factors such as nutritional status, age, gender, smoking status, physical fitness levels, and season of the study (45, 46). Of these, vitamin K status is especially important because vitamin K is a cofactor for γ-glutamyl carboxylase responsible for γ-carboxylation of OC, thus vitamin K reduces the release of OC into the general circulation while increasing calcification of the bone (24, 25, 47). An inverse relationship between [OCuc] and vitamin K status has been reported in both human and animal studies (45-49), and consequently [OCuc] has frequently been used as a sensitive marker of vitamin K nutritional status (50). However, a recent study by Yoshida et al. (51), observed that high vitamin K1 (phylloquinone) intake is associated with increased insulin sensitivity and better glycemic status in the fed state. This observation further complicates the complex crosstalk between vitamin K, [OCuc], and glucose metabolism as it was expected that vitamin K would not positively influence glucose metabolism since it promotes γ-carboxylation of OC and thus decreases [OCuc]. Another vitamin to consider with regard to bone metabolism is vitamin D, which is known to affect osteoblasts. The biologically active and most widely used marker of vitamin D status is 1a,25-dihydroxyvitamin D3 (1a,25-(OH)2D3) (52). It is believed that 1a,25-(OH)2D3 positively influences differentiation and mineralization of osteoblasts via the vitamin D receptor on osteoblasts (53, 54). Although this relationship has not been further investigated, previous studies reported that 1a,25-(OH)2D3 increased overall OC production and showed an inverse correlation between [OCuc] and 1a,25-[OH]2D3 (46, 52-55), suggesting that 1a,25-(OH)2D3 has a bimodal effect on bone health and OCuc-mediated energy metabolism. Well-controlled studies on the crosstalk between vitamins, [OC], [OCuc], and energy metabolism are needed to better understand the relationship between bones and energy metabolism.
The reciprocal relationship between bones and energy metabolism that arose from the recent identification of novel metabolic roles of OCuc has excited integrative physiologists, especially those in osteology and endocrinology. This review mainly discusses this reciprocal relationship, or feedback loop, from a hormonal regulation perspective, and reviews newly discovered metabolic functions of osteocalcin. Leptin exerts antagonistic bimodal effects on bone remodeling through two distinct hypothalamic pathways that function via the sympathetic nervous system and CART, thereby acting directly on OC-secreting osteocytes, or osteoblasts. Conversely, bones participate in energy metabolism through hormonal regulation whereby OC within the bones is released into the circulation in the form of OCuc to increase energy expenditure, increase insulin secretion capacity by enhancing pancreatic β cell proliferation, increase insulin sensitivity by increasing adiponectin secretion, and reduce fat mass. This revolutionary discovery inspired Wolf (18) to propose that the large surface of the skeleton represents an excellent site of hormone synthesis. It is noteworthy that the osteoblast-specific phosphatase encoded by the Esp gene, OST-PTP, plays a critical role in this hormonal regulation. The serendipitous finding that mice lacking an osteoblast specific protein are abnormally obese has led to fundamental progress in related fields and addressed many research questions; however, many questions remain. First of all, the role of leptin in bone remodeling in humans is not yet clearly defined despite clarity in animal studies. The association between obesity and leptin resistance (56) explains the paradoxical observations that obese individuals generally have a lower prevalence of osteoporosis when compared with non-obese individuals and that leptin secretion is proportional to fat mass. However, inconsistent associations between serum leptin levels and bone mineral density from human studies have been reported (positive [57, 58]; negative [59-61]; or no association [62-64]) and the association between intracerebral leptin levels and bone mineral density in humans has not been investigated yet. Therefore, data obtained to date complicates interpretation of the effects of leptin on bone biology in humans and emphasizes the necessity for further investigations. Secondly, while positive influences of the skeleton on energy metabolism are causal in animal models, this remains unproven in humans although a positive correlation between OC and energy homeostasis was reported in very recent human studies. With respect to future research topics, Lee and Karsenty proposed identification of the OC receptor, the nature of OC regulation, and the interactions between this novel pathway and other pathways regulating energy metabolism (65). Other research topics include elucidating the crosstalk between nutritional status, OC, and energy metabolism, and investigating the contribution of OC in the development of treatments for components of metabolic syndrome such as obesity and both type I and II diabetes, and sarcopenia. Thorough and objective research on these topics is expected in related scientific fields over the following years.
Figures and Tables
References
1. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000. 289:1504–1508.
2. Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-1 pathway. Endocrinology. 2007. 148:3419–3425.
3. Lorentzon M, Landin K, Mellström D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006. 21:1871–1878.
4. Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf). 2001. 55:341–347.
5. Laharrague P, Larrouy D, Fontanilles AM, Truel N, Campfield A, Tenenbaum R, Galitzky J, Corberand JX, Pénicaud L, Casteilla L. High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J. 1998. 12:747–752.
6. Kim GS, Hong JS, Kim SW, Koh JM, An CS, Choi JY, Cheng SL. Leptin induces apoptosis via ERK/cPLA2/Cytochrome c pathway in human bone marrow stromal cells. J Biol Chem. 2003. 278:21920–21929.
7. Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA. 2008. 105:20529–20533.
8. Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002. 17:200–209.
9. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002. 111:305–317.
10. Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA. 2004. 101:3258–3263.
11. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000. 100:197–207.
12. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005. 434:514–520.
13. Singh MK, Elefteriou F, Karsenty G. Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. Endocrinology. 2008. 149:3933–3941.
14. Takeda S. Central control of bone remodeling. Biochem Biophys Res Commun. 2005. 328:697–699.
15. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999. 140:1630–1638.
16. Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004. 117:387–398.
17. Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998. 393:72–76.
18. Wolf G. Energy regulation by the skeleton. Nut Rev. 2008. 66:229–233.
19. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996. 382:448–452.
20. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007. 130:456–469.
21. Mauro LJ, Olmsted EA, Skrobacz BM, Mourey RJ, Davis AR, Dixon JE. Identification of a hormonally regulated protein tyrosine phosphatase associated with bone and testicular differentiation. J Biol Chem. 1994. 269:30659–30667.
22. Chengalvala MV, Bapat AR, Hurlburt WW, Kostek B, Gonder DS, Mastroeni RA, Frail DE. Biochemical characterization of osteo-testicular protein tyrosine phosphatase and its functional significance in rat primary osteoblasts. Biochemistry. 2001. 40:814–821.
23. Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009. 94:827–832.
24. Bugel S. Vitamin K and bone health in adult humans. Vitam Horm. 2008. 78:393–416.
25. Maillard C, Berruyer M, Serre CM, Dechavanne M, Delmas PD. Protein-S, a vitamin K-dependent protein, is a bone matrix component synthesized and secreted by osteoblasts. Endocrinology. 1992. 130:1599–1604.
26. Berkner KL. The vitamin K-dependent carboxylase. Annu Rev Nutr. 2005. 25:127–149.
27. Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG Jr, Chua SC Jr, Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008. 183:1235–1242.
28. Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004. 89:4904–4909.
29. Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Dawson-Hughes B, Wilson PW, Cupples LA, Kiel DP. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. 2003. 77:512–516.
30. Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res. 1994. 9:1591–1595.
31. Tsugawa N, Shiraki M, Suhara Y, Kamao M, Ozaki R, Tanaka K, Okano T. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008. 26:79–85.
32. Seibel MJ, Robins SP, Bilezikian JP. Serum undercarboxylated osteocalcin and the risk of hip fracture. J Clin Endocrinol Metab. 1997. 82:717–718.
33. Hari Kumar KV, Muthukrishnan J, Verma A, Modi KD. Correlation between bone markers and bone mineral density in postmenopausal women with osteoporosis. Endocr Prac. 2008. 14:1102–1107.
34. Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Nat Acad Sci USA. 2008. 105:5266–5270.
35. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004. 350:664–671.
36. Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005. 115:3587–3593.
37. Rabøl R, Svendsen PF, Skovbro M, Boushel R, Haugaard SB, Schjerling P, Schrauwen P, Hesselink MK, Nilas L, Madsbad S, Dela F. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism. 2009. 58:1145–1152.
38. Hood D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009. 34:465–472.
39. Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 2005. 35:473–483.
40. Pietschmann P, Schernthaner G, Woloszczuk W. Serum osteocalcin levels in diabetes mellitus: analysis of the type of diabetes and microvascular complications. Diabetologia. 1988. 31:892–895.
41. Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, Ravussin E. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995. 80:1194–1202.
42. Dobnig H, Piswanger-Sölkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, Maier E, Maritschnegg P, Sieberer C, Fahrleitner-Pammer A. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab. 2006. 91:3355–3363.
43. Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008. 396:66–69.
44. Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellström D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009. 24:785–791.
45. Nimptsch K, Hailer S, Rohrmann S, Gedrich K, Wolfram G, Linseisen J. Determinants and correlates of serum undercarboxylated osteocalcin. Ann Nutr Metab. 2007. 51:563–570.
46. O'Connor E, Mølgaard C, Michaelsen KF, Jakobsen J, Lamberg-Allardt CJ, Cashman KD. Serum percentage undercarboxylated osteocalcin, a sensitive measure of vitamin K status, and its relationship to bone health indices in Danish girls. Br J Nutr. 2007. 97:661–666.
47. Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tanaka K, Okano T. Vitamin K status of healthy Japanese women: age-related vitamin K requirement for γ-carboxylation of osteocalcin. Am J Clin Nutr. 2008. 83:380–386.
48. Nimptsch K, Nieters A, Hailer S, Wolfram G, Linseisen J. The association between dietary vitamin K intake and serum undercarboxylated osteocalcin is modulated by vitamin K epoxide reductase genotype. Br J Nutr. 2009. 101:1812–1820.
49. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996. 382:448–452.
50. Sokoll LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K status in a healthy adult population. Am J Clin Nutr. 1996. 63:566–573.
51. Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr. 2008. 88:210–215.
52. van Driel M, Koedam M, Buurman CJ, Roelse M, Weyts F, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006. 99:922–935.
53. Miyahara T, Simoura T, Osahune N, Uchida Y, Sakuma T, Nemoto N, Kozakai A, Takamura T, Yamazaki R, Higuchi S, Chiba H, Iba K, Sawada N. A highly potent 26,27-Hexafluoro-1a,25-dihydroxyvitamin D3 on calcification in SV40-transformed human fetal osteoblastic cells. Calcif Tissue Int. 2002. 70:488–495.
54. van Leeuwen JP, van Driel M, van den Bemd GJ, Pols HA. Vitamin D control of osteoblast function and bone extr acellular matrix mineralization. Crit Rev Eukaryot Gene Expr. 2001. 11:199–226.
55. Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. 1993. 91:1769–1774.
56. Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996. 348:159–161.
57. Iwamoto I, Douchi T, Kosha S, Murakami M, Fujino T, Nagata Y. Relationships between serum leptin level and regional bone mineral density, bone metabolic markers in healthy women. Acta Obstet Gynecol Scand. 2000. 79:1060–1064.
58. Odabaşi E, Ozata M, Turan M, Bingöl N, Yönem A, Cakir B, Kutlu M, Ozdemir IC. Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol. 2000. 142:170–173.
59. Sato M, Takeda N, Sarui H, Takami R, Takami K, Hayashi M, Sasaki A, Kawachi S, Yoshino K, Yasuda K. Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab. 2001. 86:5273–5276.
60. Blum M, Harris SS, Must A, Naumova EN, Phillips SM, Rand WM, Dawson-Hughes B. Leptin, body composition and bone mineral density in premenopausal women. Calcif Tissue Int. 2003. 73:27–32.
61. Oh KW, Lee WY, Rhee EJ, Baek KH, Yoon KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf). 2005. 63:131–138.
62. Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001. 28:113–117.
63. Zhong N, Wu XP, Xu ZR, Wang AH, Luo XH, Cao XZ, Xie H, Shan PF, Liao EY. Relationship of serum leptin with age, body weight, body mass index, and bone mineral density in healthy mainland Chinese women. Clin Chim Acta. 2005. 351:161–168.
64. Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta. 2008. 387:31–35.
65. Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008. 19:161–166.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download