Abstract
Gastrinoma is the most frequent functional pancreaticoduodenal endocrine tumor in patients with multiple endocrine neoplasia type 1 (MEN 1). Primary hepatic gastrinomas in MEN 1 are very rare, with no previous reports published in the literature. We reported the case of a 39 yr old female patient with a history of repeated peptic ulcers and a hypoglycemia episode. Abdominal CT indicated a well-defined liver mass and a pancreatic head mass. Somatostatin-receptor scintigraphy with [111In] DTPA octeotride demonstrated a strong uptake of the radiotracer in the left lateral segment at the site of the hepatic mass. After operation, immunohistochemical staining was consistent with pancreatic insulinoma and primary hepatic gastrinoma. As the liver is a common site of metastases from gastrinoma, primary liver gastrinoma has not yet been reported with MEN 1. We diagnosed this patient using immunohistochemical studies and treated this patient by hepatic segmentectomy.
Multiple endocrine neoplasia type 1 (MEN 1) is a familial tumor syndrome characterizing tumors of the parathyroid glands, the enteropancreatic neuroendocrine system, the anterior pituitary gland, and the skin. The most common endocrine tumors are parathyroid tumors, which cause hyperparathyroidism and hypercalcemia. Other tumors of MEN 1 include gastrinomas, insulinomas, prolactinomas, and carcinoid tumors.
Gastrinoma is the most frequent functional pancreaticoduodenal endocrine tumor in patients with MEN 1 and a major determinant of mortality when stricken with this syndrome (1). The gastrinoma form of MEN 1 often arises from the gastrinoma triangle, a region including the cystic and common bile ducts, second and third portions of the duodenum, and the head and body of the pancreas (2). However, gastrinomas can also appear in other locations, such as the stomach, the ovaries, or the liver, although the tendency to arise from these locations is infrequent (3). Here we report the case of woman with MEN1 arising as primary hepatic gastrinoma.
A 39-yr-old woman with a 1-yr history of several hospital admissions resulting from abdominal pain, nausea, and vomiting and who had a primary closure and omental patch operation for an acute surgical abdomen resulting from a spontaneous perforation of the duodenum a year ago was admitted to hospital because of a change in mental status. The proton pump inhibitor pantoprazole proved curative for many of her gastrointestinal symptoms during this 1-yr time period. Additional medical history included a craniotomy in 1980, having resulted from the diagnosis of pituitary macroadenoma.
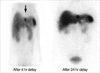
Laboratory results upon admission demonstrated a low blood glucose level (30 mg/dL), while 4 hr fasting blood glucose was 30 mg/dL, plasma insulin was 15.0 uIU/mL and C-peptide was 4.16 ng/mL. Computed tomography (CT) of the abdomen demonstrated a 1.5×1.2 cm size hypervascular mass in head portion of the pancreas (Fig. 1A) and a hypervascular mass in the lateral segment of the liver (Fig. 1B). On admission, the fasting plasma level of gastrin was 447.0 pg/mL (0-110 pg/mL). Somatostatin-receptor scintigraphy with [111In] DTPA octreotide showed strong uptake of the radiotracer in the left lateral segment at the site of the hepatic mass (Fig. 2). In addition, laboratory studies demonstrated elevated serum calcium (12.1 mg/dL, 8.4-10.2 mg/dL) and elevated PTH (233.3 pg/mL, 8-76 pg/mL) was observed. Neck CT demonstrated a 2×1 cm soft tissue mass in dorsal portion of left thyroid, compatible with parathyroid adenoma and right thyroid nodule. A parathyroid Tc-99m MIBI scan demonstrated a 2×1 cm and a 1 cm hot uptake lesions at the left and right thyroid gland inferior pole.
The patient underwent parathyroidectomy of the left both and right upper organ segments.
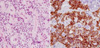
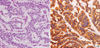
Half of right lower segment was remained. Pathological examination indicated parathyroid hyperplasia. The patient was treated by Whipple's operation. Intraoperative ultrasound demonstrated a hypoechoic mass in pancreas head and in the left lateral segment of the liver. The mass was not demonstrated in any other portion of pancreas or liver. Microscopic examination demonstrated a well-differentiated endocrine tumor of the pancreas head of 1.6×1.5×0.9 cm. The patient underwent hepatic segmentectomy of the lateral segment, which demonstrated a well-differentiated endocrine tumor measuring 1.6×1.5×0.9 cm in size. Immunohistochemical studies of the pancreas head tumor cells were positive for chromogranin A, synaptophysin, and insulin, while negative for gastrin, glucagon (Fig. 3). Immunohistochemical studies of the liver tumor cells were positive for gastrin, while negative for glucagon, insulin, and somatostatin (Fig. 4). Post-operatively, the patient's serum gastrin normalized to 34.6 pg/mL (0-110 pg/mL) rapidly, fasting blood glucose normalized to 85 mg/dL, and fasting insulin level decreased to normal at 3.8 uIU/mL (4-16 uIU/mL). In addition, the patient's abdominal pain disappeared and she did not exhibit neuroglycopenic symptoms. The patient was negative for further symptoms at 3-month intervals and had no symptoms at 1-yr postoperative.
Based on these results, we concluded that the patient had primary hyperparathyroidism, pituitary adenoma, pancreatic insulinoma, and primary hepatic gastrinoma, resulting from MEN 1.
The vast majority of patients with MEN 1 display heterozygotic germline mutations of the MEN 1 gene. The patient had no familial history of endocrine tumors. Although genetic evaluations of the patient's family members were not performed, we will analyze clinical manifestations of MEN 1 if and when they occur in her family members.
It is often a question in clinical and pathological practice, whether liver tumors of unknown primary origin represent metastases or whether they are primary to the liver. Approximately 20% of gastrinomas occur as part of the inherited tumor syndrome MEN 1. Most gastrinomas are located in the 'gastrinoma triangle', comprising the head of the pancreas, and the first and second parts of the duodenum (4). In rare occasions, ectopic gastrinomas appear in other locations, such as the stomach, the ovaries, or the liver; there have been 10 reports of primary hepatic gastrinoma identified in the literature (3,5). Primary hepatic gastrinoma tends to show the following qualities: occurrence in a slightly younger patient population as compared with patients with other Zollinger Ellison Syndrome (ZES) tumors, demonstrating a predilection for male patients, and a lack of association with the MEN 1 syndrome (6). To our knowledge, this is the first case of diagnosed primary hepatic gastrinoma in an MEN 1 patient.
In this report, our patient present with symptoms of hypoglycemia secondary to excessive secretion of insulin and had symptoms suggestive of severe peptic ulcer disease, based on increased gastrin levels. After performing a search on MEN 1 syndrome, we gave a diagnosis of hyperparathyroidism, insulinoma of pancreas, and primary hepatic gastrinoma. Based on the patient's past medical history of visual disturbance, amenorrhea, and pituitary craniectomy, we suggested she had prolactinoma. The diagnosis of primary hepatic gastrinoma was made based on both preoperative and intraoperative imaging studies for the location of the primary tumor, which included CT scan, somatostatin-receptor scintigraphy, intraoperative ultrasound, and failed to reveal any abnormalities in the pancreas or duodenum. Immunohistochemistry studies performed after hepatectomy revealed hepatic gastrinoma. Immunohistochemical analyses of specimens obtained from the Whipple's operation performed on the patient revealed insulinoma of the pancreatic head. We believe that the disappearance of all clinical manifestations after the operation substantiates the evidence of primary hepatic gastrinoma.
Somatostatin-receptor scintigraphy has the advantage of identifying tumors functionally, based on the size of the tumor mass and the concentration of somatostatin receptors. The disadvantage of somatostatin-receptor scintigraphy is the lack of anatomic specificity. Nuclear medicine imaging, even with Single photon emission computed tomography (SPECT) cross-sectional imaging, provides only general anatomic definition of surrounding organs. Somatostatin-receptor scintigraphy should be combined with CT scan or other anatomic imaging techniques to provide anatomic correlates to the scintigraphy findings (7).
The management of gastrinoma of MEN 1 is controversial (8). Some have advocated a non-operative approach to gastrinoma by controlling the effects of excess gastrin with protonpump inhibitors. Some recommend an operative approach if the tumor reaches 3 cm in size, the size at which the risk of liver metastases increases significantly. The present authors recommend a total surgical resection of the gastrin-producing tumor in the pancreas or duodenum, including dissection of the regional lymph nodes. Symptom control can be achieved by administration of highly potent proton pump inhibitors, in up to 2-3 fold standard doses to inhibit gastric acid hypersecretion (9). Disagreement exists not only about the indication for surgical exploration but also about the type of operation, especially whether distal pancreatectomy or pancreaticoduodenectomy should be preferred (4). We performed a hepatic segmentectomy on our patients, which corrected the patient's overestimated gastrin level; abdominal pain disappeared without the use of proton pump inhibitors.
Long-term survival rates seem to be determined by the possibility of complete resection of the primary tumor by the presence of the MEN 1 syndrome (10, 11). Due to this unprecedented case of primary hepatic gastrinoma in MEN 1, the prognosis was unpredictable. However, a resection of the gastrinoma and insulinoma with Whipple's operation and a hepatic segmentectomy of the lateral segment provide a better prognosis in this patient.
Figures and Tables
Fig. 1
CT scan of abdomen, (A) Arrowhead points to 1.5×1.2 cm size hypervascular mass in the pancreas head portion. (B) Arrow showed hypervascular mass at liver lateral segment.
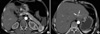
Fig. 2
Somatostatin-receptor scintigraphy with [111In] DTPA octreotide showed strong uptake of the radiotracer in the left lateral segment at the site of the hepatic mass.
References
1. Bartsch DK, Langer P, Rothmund M. Surgical aspects of gastrinoma in multiple endocrine neoplasia type 1. Wien Klin Wochenschr. 2007. 119:602–608.
2. Zhou H, Schweikert HU, Wolff M, Fischer HP. Primary peripancreatic lymph node gastrinoma in a woman with MEN1. J Hepatobiliary Pancreat Surg. 2006. 13:477–481.
3. Díaz R, Aparicio J, Pous S, Dolz JF, Calderero V. Primary hepatic gastrinoma. Dig Dis Sci. 2003. 48:1665–1667.
4. Fendrich V, Langer P, Waldmann J, Bartsch DK, Rothmund M. Management of sporadic and multiple endocrine neoplasia type 1 gastrinomas. Br J Surg. 2007. 94:1331–1341.
5. Wu PC, Alexander HR, Bartlett DL, Doppman JL, Fraker DL, Norton JA, Gibril F, Fogt F, Jensen RT. A prospective analysis of the frequency, location and curability of ectopic (nonpanceaticoduodenal non-nodal) gastrinoma. Surgery. 1987. 122:1176–1182.
6. Moriura S, Ikeda S, Hirai M, Naiki K, Fujioka T, Yokochi K, Gotou S. Hepatic gastrinoma. Cancer. 1993. 72:1547–1550.
7. Doherty GM. Multiple endocrine neoplasia type 1. J Surg Oncol. 2005. 89:143–150.
8. MacFarlane MP, Fraker DL, Alexander HR, Norton JA, Lubensky I, Jensen RT. Prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia type 1. Surgery. 1995. 18:973–979.
9. Banasch M, Schmitz F. Diagnosis and treatment of gastrinoma in the era of proton pump inhibitors. Wien Klin Wochenschr. 2007. 119:573–578.
10. Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors, causes of death and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999. 17:615–630.
11. Weber HC, Venzon DJ, Lin JT, Fishben VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: syndrome: a prospective long-term study. Gastroenterology. 1995. 108:1637–1649.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download