Abstract
Cochinchina momordica seed is the dried ripe seed of Momordica cochinchinensis, a perennial vine. The antiulcer effect of an extract from cochinchina momordica seeds (SK-MS10) was evaluated in a rat model of acetic acid-induced gastric ulcers. Gastric ulcers were produced by subserosal injection of acetic acid. SK-MS10 (200 mg/kg) or vehicle was administered orally once per day for 14 days after the acetic acid injection. The stomach was removed and the ulcer size measured at day 7 and 14 of the treatment. Expression of vascular endothelial growth factor (VEGF) was assessed by real-time RT-PCR and Western blot analysis. In addition, the microvasculature density (MVD) adjacent to the ulcer margin was examined by immunohistochemistry. The treatment with SK-MS10 for 7 and 14 days significantly accelerated ulcer healing and increased the expression of mRNA (at day 7) as well as VEGF protein (at day 14) compared to the vehicle-treated rats. The MVD for factor VIII was also higher in the SK-MS10 treatment group compared to the vehicle-treated rats; however, these differences were not statistically significant. These results suggest that SK-MS10 treatment accelerates the healing of gastric ulcers via upregulation of VEGF and angiogenesis in an acetic acid rat model.
Peptic ulcers affect a large portion of the population worldwide and are commonly induced by Helicobacter pylori infection or one of several other factors including stress, smoking, and the ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) (1, 2). Currently, the relatively higher incidence of gastric ulcers in the elderly and the expanded use of NSAIDs, along with alcohol ingestion and stress have been shown to increase with aging (3). The approach to the treatment of peptic ulcer disease includes inhibition of gastric acid secretion by H2 receptor blockers or proton pump inhibitors (PPIs) as well as eradication of H. pylori. However, development of tolerance to such treatments and the incidence of relapse as well as side effects interfere with their clinical usefulness. Therefore, new antiulcer drugs are under investigation.
Cochinchina momordica is the dried ripe seed of Momordica cochinchinensis, a perennial vine that grows in southern China and Vietnam, and is known for its anti-inflammatory activity against suppurative skin infections (4). Chemical analysis shows that the cochinchina momordica seeds are composed of compounds including fatty acids, saponins, proteins, α-spinasterol, oleanolic acid, and momordica acid (4). Among these compounds, momordica saponin I, glycoside, a triterpenoid saponin containing disaccharide chain, has been found to be a major active ingredient (5). Recently, we reported that SK-MS10, an extract from cochinchina momordica seeds, has gastroprotective effects against acute gastric mucosal damage by suppressing proinflammatory cytokines, down-regulating cytosolic phospholipase A2 (cPLA2), 5-lipoxygenase (5-LOX), and increasing the synthesis of mucus in an acute gastric mucosal damage model, using ethanol and water immersion restraint stress (WRS) (6). Furthermore, we demonstrated that the calcitonin gene-related peptide (CGRP)-nitric oxide (NO) pathway played an important role in the gastroprotective effects of SK-MS10 (6).
The so-called acetic acid ulcer model has been shown to be a useful model for investigating the pathophysiology of gastric ulcers and the efficacy of antiulcer drugs (7). This model mimics human ulcers in terms of both pathophysiological features and healing mechanisms. To investigate whether SK-MS10 improves the healing of gastric ulcers, and to evaluate the mechanisms involved, we used the acetic acid-induced ulcer rat model in this study. The goal of this study was to investigate the effects of SK-MS10 on angiogenic responses such as the microvasculature density, and the expression of vascular endothelial growth factor (VEGF).
Five liter of aqueous ethanol solution was added to one kg (dry weight) of cochinchina momordica, purchased at an herb market in Korea. Extraction was performed for 4 hr at 80℃, and this process was performed twice. The extract was filtered and concentrated under reduced pressure at 60℃ using a rotary evaporator. After complete removal of the solvent in a vacuum oven, 60 g of ethanol extract in powder form (SK-MS10) was obtained. SK-MS10 was dissolved in the carboxymethyl-cellulose (CMC) during the experiment.
Seven-week-old male Spraque-Dawley rats (Orient Co. Ltd., Seoul, Korea) were housed in a cage maintained at 23℃, 12/12-hr light/dark cycles under specific pathogen-free conditions. After 1 week of adaptation, the 8-week-old rats weighing 250-300 g were used for the experiments. The rats were starved but allowed water for 12 hr prior to the experiments. All experimental procedures described here were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (SNU-070419-2).
Gastric ulcers were induced with acetic acid treatment, according to a previously described method (8). Briefly, the fasting rats were anesthetized with an intramusclular injection of 80 mg/kg of ketamine, and then their stomachs were exposed via a midline incision. Acetic acid (20%, 30 µL) was injected into the subserosal layer at the junction of the anterior wall of the antrum and corpus using a microsyringe (Hamilton Co., Reno, NV, USA). The abdominal incision was then sutured closed. From day 1 after ulcer induction, the rats were treated with SK-MS10 (200 mg/kg) or a vehicle by gavage. The rats were sacrificed on day 7 or 14. Each experimental group consisted of six animals. After measuring the size of the ulcer, the ulcer tissue was cut in half. One half was used for histological and immunohistochemical examination, while the other was used for mRNA level assays. As soon as the mucosa was collected, it was stored in liquid nitrogen and the samples were kept in a -80℃ freezer until used for the experiments.
After the animals were sacrificed, the isolated stomachs were cut open along the greater curvature and washed in ice-cold saline. To assess the degree of gross mucosal damage, the mucosal sides of the stomachs were photographed using a digital camera, and part of the mucosa was immediately fixed with a 10% formalin solution. After fixed in formalin overnight, the stomach was opened along the greater curvature and spread out with pins on a cork board, and then photographed. The ulcerated area (mm2) was quantified using the following equation: S=π (d1/2)×(d2/2), where S represented the ulcerated area (mm2), d1 and d2 the longest longitudinal and transverse diameters of the ulcer.
For the angiogenesis studies, the sections were incubated with an antibody for the von Willebrand factor (factor VIII-related endothelial antigen; Chemicon International, Temecula, CA, USA) after deactivation of endogenous peroxidase with 0.3% H2O2 and blocking of nonspecific binding sites. The microvasculature was visualized by the avidin-biotin-peroxidase complex method. The degree of microvasculature found in the ulcer base granulation tissue was determined in three randomly chosen 1 mm2 fields. The microvasculature density was expressed as the number of vessels per mm2 of the ulcer base.
The gastric mucosa was homogenized with lysis buffer containing 25 mM Tris-HCl (pH 7.4), EGTA (1 mM), DTT (1 mM), leupeptin (10 µg/mL), aprotinin (10 µg/mL), PMSF (1 mM), and Triton X-100 (0.1%). Briefly, the proteins (each sample, 30 µg) were separated by SDS-PAGE (7.5% wt/wt gel) and transferred to nitrocellulose membranes. All procedures were carried out in Tris buffer (40 mM, pH 7.55) containing 0.3 M of NaCl and 0.3% Tween 20. The membranes were then blocked with dried milk (6% wt/vol), and subsequently incubated with VEGF antibody (mouse monoclonal antibody, 1:500; Chemicon International) at 4℃ overnight. The blots were incubated with secondary antibody (goat antimouse polyclonal antibody, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and an imaging analyzer was used to measure the band densities.
RNA was extracted from the gastric mucosa using the RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RNA samples were diluted to a final concentration of 0.5 mg/mL in RNase-free water and stored at -80℃ until use. Synthesis of the cDNA was performed with 1 mg of total RNA with M-MLV Reverse Transcription Reagents (Invitrogen, Carlsbad, CA, USA). The 20-µL reverse transcription reaction consisted of 4 µL of First-strand buffer, 500 mM deoxynucleoside triphosphate mixture, 2.5 mM oligo (dT)12-18 primer, 0.4 U/mL ribonuclease inhibitor, and 1.25 U/mL Moloney murine leukemia virus reverse transcriptase (Invitrogen). The thermal cycling parameters for the reverse transcription were 5 min at 65℃, 50 min at 37℃ and 15 min at 70℃. Real time PCR amplification and determination were performed using the ABI PRISM 7000 Sequence Detection System, TaqMan universal PCR master mix, commercially available predesigned, gene specific primers, and FAM-labelled probe sets for quantitative gene expression (TaqMan Gene Expression Assays, rodent VEGF, mouse β-actin; Applied Biosystems, Foster City, CA, USA). All of the probes used in these experiments spanned an exon-intron boundary. The VEGF and β-actin mRNA was quantified by parallel estimation. The thermal cycler conditions were 2-min hold at 50℃ and 10-min hold at 95℃, followed by 40 cycles of 15 s at 95℃ and 1 min at 60℃.
All statistical calculations were performed using SPSS software (version 12.0; SPSS Inc., Chicago, IL, USA). The results were compared using the Mann-Whitney U test and the Wilcoxon rank sum test. All values are reported as means±standard errors. Statistical significance was set at P value <0.05.
SK-MS10 significantly accelerated ulcer healing by day 7 and day 14. That is, compared to the vehicle-treated group (Fig. 1A, B) the mean ulcer size in the SK-MS10-treated group (Fig. 1C, D) was significantly smaller by day 7 and 14 after ulcer induction. Numerically, the ulcer area 7 and 14 days after SK-MS10 treatment was 33.2 mm2 and 9.3 mm2, respectively, which was smaller than the 52.6 mm2 and 32.3 mm2 size of the vehicle treated group (Fig. 1E).
As shown in Fig. 2, microvessels were stained brown using the von Willebrand factor antibody. The microvasculature density (MVD) in the SK-MS10 treated group was increased compared to the vehicle treated group; however, this difference did not reach statistical significance. That is, the microvessel densities in the ulcer granulation tissues of the SK-MS10 treated rats on day 7 and 14 were 40.8 vessels/mm2 and 36.8 vessels/mm2, respectively, which was higher than in the vehicle treated rats (32.4 vessels/mm2 and 24.2 vessels/mm2, respectively).
The mRNA expression of VEGF after 7 days of SK-MS10 treatment was significantly higher than in the vehicle treated group (0.7 vs. 5.4 for VEGF/β-actin, respectively Fig. 3A). However, the mRNA expression of VEGF after 14 days of SK-MS10 treatment was not significantly different from the vehicle treated group (Fig. 3A). On Western blot analysis, the expression of VEGF proteins 14 days after SK-MS10 treatment was significantly higher than in the vehicle group (2.7 vs. 6.0 for VEGF/β-actin, respectively Fig. 3B). However, the protein expression of VEGF 7 days after SK-MS10 treatment was not significantly different from the vehicle treated group (Fig. 3B).
Since introduced in 1969 by Takagi et al. (9), the acetic acid-induced gastric ulcer model has proved useful for investigating the pathophysiology of gastric ulcer disease and the efficacy of antiulcer drugs (7). The reasons for the usefulness of this model include the following. First, the ulcer induction procedure is simple, readily resulting in ulcers of consistent size and severity with a 100% success rate. Second, the acetic acid-induced ulcers resemble human peptic ulcers both macroscopically and histologically. Indeed, spontaneous relapse of healed ulcers is frequently observed, just as in patients with peptic ulcer disease. Finally, the ulcers respond well to various anti-ulcer drugs, such as PPI, sucralfate and several growth factors. In this study, we investigated the effects of SK-MS10 on the healing of this type of experimental ulcer in the rat, and found that oral administration of SK-MS10, at a dose of 200 mg/kg for 7 and 14 days, accelerated the healing of the gastric mucosa in the rat model.
The regrowth of blood vessels into the ulcerated area, i.e., angiogenesis, plays a pivotal role in the acceleration of the healing of ulcers since the neovasculature promotes nutrient supply to the healing tissue (10). This complex sequence of events requires a high degree of coordination among different cell types, which is regulated by several factors. Among them, growth factors such as the vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and their receptors are known to play an important role in tissue repair and the healing of ulcers (11, 12). VEGF, previously described as a vascular permeability factor, is also known as a growth factor that is involved in the mucosal protection and the angiogenic response that occurs during the healing of ulcers (13). Specific expression of VEGF has been demonstrated at the ulcer margin in humans (14). In addition, exogenous VEGF has been reported to significantly enhance the healing of acute gastric mucosal injury induced by ethanol and duodenal ulceration (15). Furthermore, local injection of plasmid-DNA encoding VEGF has been shown to stimulate the healing of ulcers in the rat (16). By contrast, NSAIDs, cyclooxygenase-2 (COX-2) inhibitors, and alendronate have been shown to delay the healing of ulcers, and impair angiogenesis and the down regulation of growth factors such as bFGF and VEGF (17-19). Recently, the saponins isolated from Red Ginseng exhibited wound healing of burns in mice and promoted angiogenesis via the stimulation of VEGF production (20). In the present study we found that SK-MS10, with momordica saponin as a major component, enhanced the expression of VEGF by stimulation of angiogenesis in the gastric mucosa of the rat, similar to the effects of Red Ginseng. This expression of VEGF has been reported to be upregulated by COX-2 derived prostaglandin E2 (PGE2) (21). Thus, it might be valuable to investigate whether SK-MS10 could induce COX-2/PGE2 production in acetic acid induced ulcer. Besides some bioactive components, cochinchina momordica are also rich in protease inhibitors. Several trypsin inhibitors and chymotrypsin inhibitor have been isolated (22-25). Protease inhibitor from cochinchina momordica has been reported to possess antioxidative activities in the rat hepatocyte (26). In addition, high carotinoid contents such as carotene, lycophene are known to be detected in the active fraction of cochinchina momordica seed membrane (27). Carotenoids also are known to potent antioxidative activity and have been reported to have anti-ulcerogenic effect on ulcer models in rat (28). These antioxidative components of the seeds may provide a favorable environment for ulcer healing and the anti-ulcerogenic effect. However, further studies with these isolates from cochinchina momordica and phytochemical analysis are needed to clarify the main contributor of the mechanism on ulcer healing. Interestingly, the duration of treatment for the detection of a significant difference was different for the mRNA and the protein expression. That is, the mRNA expression of VEGF 7 days after SK-MS10 treatment, and the expression of VEGF proteins 14 days after SK-MS10 treatment were significantly higher than in the control group. This discrepancy might be due to the lag time between the translation of mRNA and the expression of the protein. In addition, it is possible that the VEGF expression is regulated at the post-translational level, although further studies are necessary to clarify this.
MVD assessment is the most commonly used technique to quantify angiogenesis. Initially, we used antibodies directed against platelet endothelial cell adhesion molecules, CD31 and CD34; commonly used for the assessment of angiogenesis in several prior studies (29-31). However, these antibodies did not work properly for staining the microvessels even after several trials. As an alternative marker, we used an antibody against the factor VIII-related antigen, also known as the von Willebrand factor, staining mainly the mature vessels and cross reacting lymphatic endothelium (32). This marker, in the SK-MS10 treated group, was increased compared to the vehicle treated group; however, the difference did not reach statistical significance. The relatively small sample size (n=6) might have limited the power to detect a significant difference between the two groups. This finding may also be partly due to the variation in the assessment of the MVD, depending on the fields examined, and the experience of the pathologist. So, further study extending the number of animals is needed to clarify the effect on the angiogenesis of SK-MS10 for strengthening the statistical power. However, the increasing tendency of the MVD with SK-MS10 treatment was supported by the results of enhanced expression of VEGF by using the real-time PCR and Western blot analysis.
Several studies have provided evidence for a role of sensory neuron in gastric ulcer healing. Sensory nerve ablation by high dose capsaicin impaired the ulcer healing (33). Recently, Ohno et al. has demonstrated that CGRP had proangiogenic activity associated with the enhancement of ulcer healing using CGRP knockout mice (30). We previously reported that SK-MS10 has a gastroprotective effect against acute gastric mucosal damage using a model with ethanol and WRS (6). These gastroprotective effects were found to be mediated by the upregulation of CGRP. Thus CGRP might play a role in the ulcer healing by SK-MS10 in the acetic acid-induced ulcer model. Actually CGRP has been known to be the major transmitter from afferent nerve fibers and suppress acid output (34). Thus, there is a possibility of the stimulation of CGRP by SK-MS10 could contribute to the antisecretory effect. By contrast, proinflammatory cytokines play an important role in impairing ulcer healing (19, 35). IL-1β has been used in a model of induction of gastric ulcer recurrence; approximately 80-100% of healed ulcers recur at the sites of scarred mucosa within 48 h after injection of IL-1β (36). In a previous study, we demonstrated that SK-MS10 reduced the increases of mucosal myeloperoxidae, IL-1β, and TNFα levels in an acute mucosal damage model using ethanol and WRS (6). This result further suggested that SK-MS10 have potent inhibitory effect on inflammation, which may also contribute to the enhancement of ulcer healing. In addition to this gastroprotective effect of SK-MS10 we identified an additional mechanism of VEGF expression associated with the anti-ulcer effects of SK-MS10 in the chronic ulcer model using acetic acid in the rat.
In conclusion, the results of this study suggest that SK-MS10 accelerates the healing of acetic acid induced gastric ulcers in rats by enhancing angiogenesis and the expression of the angiogenic growth factor, VEGF. These findings suggest that SK-MS10 might be an alternative treatment of gastric ulcer disease in humans sometime in the near future. In addition, acetic acid ulcer models appear to be quite useful for pharmacological studies of anti-ulcer drug.
Figures and Tables
Fig. 1
Effects of SK-MS10 on the healing of gastric ulcers. Macroscopic appearance of ulcers generated at the gastric mucosa in the vehicle treated group at 7 and 14 days (A, B) and the SK-MS10 treated group at 7 and 14 days (C, D). Arrows indicate ulcer. (E) Summarized results on changes of the ulcer area in the vehicle and SK-MS10 treated groups. Results are the mean±SE in 6 animals per group.
*P value <0.05 when compared with the vehicle treated group.
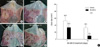
Fig. 2
Effects of SK-MS10 on angiogenesis in the gastric ulcer. Immunochemical staining of microvessels with the von Willebrand factor in the ulcer bases of rats in the vehicle treated group at 7 and 14 days (A, B) and the SK-MS10 treated group at 7 and 14 days (C, D). Note the von Willebrand factor positive cells (dark brown spots indicated by arrows). (E) The number of microvessels at the gastric ulcer bases of the rats in the vehicle and the SK-MS10 treated groups. Results are mean±SE in 6 animals per group.
*P value <0.05 when compared with the vehicle treated group.

References
1. Nash J, Lambert L, Deakin M. Histamine H2-receptor antagonists in peptic ulcer disease. Evidence for a prophylactic use. Drugs. 1994. 47:862–871.
2. Kim SY, Woo CW, Lee YM, Son BR, Kim JW, Chae HB, Youn SJ, Park SM. Genotyping CagA, VacA subtype, IceA1, and BabA of Helicobacter pylori isolates from Korean patients, and their association with gastroduodenal diseases. J Korean Med Sci. 2001. 16:579–584.
3. Ishihara M, Ito M. Influence of aging on gastric ulcer healing activities of cimetidine and omeprazole. Eur J Pharmacol. 2002. 444:209–215.

4. Gao XM. Gao XM, editor. Mu Bie Zi (Semen momordicae). Chinese Materia Medicia. 2005. Beijing: Traditional Chinese Materia Medica Press;601–602.
5. Kubota K, Sato M, Murakami T, Yamagishi T. Pharmacological studies on the saponin isolated from the seed of Momordica cochinchinensis Sprenger. Yakugaku Zasshi. 1971. 91:174–179.

6. Kang JM, Kim N, Kim B, Kim JH, Lee BY, Park JH, Lee MK, Lee HS, Jang IJ, Kim JS, Jung HC, Song IS. Gastroprotective action of cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA(2)/5-LOX Pathway. Dig Dis Sci. 2009. 54:2549–2560.
7. Okabe S, Amagase K. An overview of acetic acid ulcer models--the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005. 28:1321–1341.
8. Okabe S, Pfeiffer CJ. Chronicity of acetic acid ulcer in the rat stomach. Am J Dig Dis. 1972. 17:619–629.

9. Takagi K, Okabe S, Saziki R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn J Pharmacol. 1969. 19:418–426.

10. Szabo S, Kusstatscher S, Sakoulas G, Sandor Z, Vincze A, Jadus M. Growth factors: new 'endogenous drugs' for ulcer healing. Scand J Gastroenterol Suppl. 1995. 210:15–18.

11. Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005. 50:Suppl 1. S24–S33.

12. Jo JS, Hong SB, Shin HI, Choi JY. Homologous fibronectin enhances healing of excised wounds in rats. J Korean Med Sci. 1991. 6:197–205.

13. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989. 246:1306–1309.

14. Takahashi M, Kawabe T, Ogura K, Maeda S, Mikami Y, Kaneko N, Terano A, Omata M. Expression of vascular endothelial growth factor at the human gastric ulcer margin and in cultured gastric fibroblasts: a new angiogenic factor for gastric ulcer healing. Biochem Biophys Res Commun. 1997. 234:493–498.

15. Szabo S, Vincze A, Sandor Z, Jadus M, Gombos Z, Pedram A, Levin E, Hagar J, Iaquinto G. Vascular approach to gastroduodenal ulceration: new studies with endothelins and VEGF. Dig Dis Sci. 1998. 43:9 Suppl. 40S–45S.
16. Jones MK, Kawanaka H, Baatar D, Szabo IL, Tsugawa K, Pai R, Koh GY, Kim I, Sarfeh IJ, Tarnawski AS. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001. 121:1040–1047.

17. Amagase K, Hayashi S, Nishikawa K, Aihara E, Takeuchi K. Impairment of gastric ulcer healing by alendronate, a nitrogen-containing bisphosphonate, in rats. Dig Dis Sci. 2007. 52:1879–1889.

18. Guo JS, Cho CH, Lam Liu ES, Choy HT, Wang JY, Leung Koo MW. Antiangiogenic effect of a highly selective cyclooxygenase-2 inhibitor on gastric ulcer healing in rats. Toxicol Appl Pharmacol. 2002. 183:41–45.

19. Amagase K, Yokota M, Tsukimi Y, Okabe S. Characterization of "unhealed gastric ulcers" produced with chronic exposure of acetic acid ulcers to indomethacin in rats. J Physiol Pharmacol. 2003. 54:349–360.
20. Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol. 2006. 148:860–870.

21. Tamura K, Sakurai T, Kogo H. Relationship between prostaglandin E2 and vascular endothelial growth factor (VEGF) in angiogenesis in human vascular endothelial cells. Vascul Pharmacol. 2006. 44:411–416.

22. Huang B, Ng TB, Fong WP, Wan CC, Yeung HW. Isolation of a trypsin inhibitor with deletion of N-terminal pentapeptide from the seeds of Momordica cochinchinensis, the Chinese drug mubiezhi. Int J Biochem Cell Biol. 1999. 31:707–715.

23. Hernandez JF, Gagnon J, Chiche L, Nguyen TM, Andrieu JP, Heitz A, Trinh Hong T, Pham TT, Le Nguyen D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry. 2000. 39:5722–5730.
24. Wong RC, Fong WP, Ng TB. Multiple trypsin inhibitors from Momordica cochinchinensis seeds, the Chinese drug mubiezhi. Peptides. 2004. 25:163–169.

25. Tsoi AY, Wong RC, Ng TB, Fong WP. First report on a potato I family chymotrypsin inhibitor from the seeds of a Cucurbitaceous plant, Momordica cochinchinensis. Biol Chem. 2004. 385:185–189.

26. Tsoi AY, Ng TB, Fong WP. Antioxidative effect of a chymotrypsin inhibitor from Momordica cochinchinensis (Cucurbitaceae) seeds in a primary rat hepatocyte culture. J Pept Sci. 2005. 11:665–668.
27. Ishida BK, Turner C, Chapman MH, McKeon TA. Fatty acid and carotenoid composition of gac (Momordica cochinchinensis Spreng) fruit. J Agric Food Chem. 2004. 52:274–279.
28. Gurbuz I, Akyuz C, Yesilada E, Sener B. Anti-ulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J Ethnopharmacol. 2000. 71:77–82.
29. Yeo M, Kim DK, Han SU, Lee JE, Kim YB, Cho YK, Kim JH, Cho SW, Hahm KB. Novel action of gastric proton pump inhibitor on suppression of Helicobacter pylori induced angiogenesis. Gut. 2006. 55:26–33.

30. Ohno T, Hattori Y, Komine R, Ae T, Mizuguchi S, Arai K, Saeki T, Suzuki T, Hosono K, Hayashi I, Oh-Hashi Y, Kurihara Y, Kurihara H, Amagase K, Okabe S, Saigenji K, Majima M. Roles of calcitonin gene-related peptide in maintenance of gastric mucosal integrity and in enhancement of ulcer healing and angiogenesis. Gastroenterology. 2008. 134:215–225.

31. Watanabe T, Higuchi K, Taira K, Sasaki E, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Arakawa T. Rebamipide reduces delay in gastric ulcer healing in cyclooxygenase-2-deficient mice. Dig Dis Sci. 2005. 50:Suppl 1. S63–S69.

32. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.
33. Ma L, Chow JY, Wong BC, Cho CH. Role of capsaicin sensory nerves and EGF in the healing of gastric ulcer in rats. Life Sci. 2000. 66:PL213–PL220.

34. Lawson DC, Mantyh CR, Pappas TN. Effect of CGRP antagonist, alpha-CGRP 8-37, on acid secretion in the dog. Dig Dis Sci. 1994. 39:1405–1408.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download