Abstract
In radiofrequency (RF) ablation for idiopathic left ventricular tachycardia (ILVT), the termination of tachycardia during RF ablation is considered a hallmark of success. However, in cases of patients with difficulty of induction of ventricular tachycardia (VT), the evaluation of procedural success can be problematic. We have observed thermal responses reflected as ventricular rhythm change to RF energy delivered on sinus rhythm for ILVT. We therefore describe the significance of repetitive ventricular responses. The study subjects were 11 ILVT patients for whom RF energy was delivered during sinus rhythm because of difficulty in re-induction of tachycardia. During each energy delivery, we focused on the occurrence of repetitive ventricular responses especially exhibiting a similar morphology to clinical VT. The repetitive ventricular responses were noted in 10 of 11 patients. Two patients received a second procedure due to the recurrence of ILVT. The mean follow-up period was 36.2±12.8 months. The clinical course of the remaining patients was favorable and without recurrence of ILVT. Based on the favorable clinical outcomes, ablation-induced repetitive ventricular responses with similar QRS morphology to clinical ILVT are useful markers for selecting an ablation site and could be used as an additional mapping method, termed as "thermal mapping".
In radiofrequency catheter ablation (RFCA) for treatment of idiopathic left ventricular tachycardia (ILVT), termination of tachycardia with the delivery of radiofrequency (RF) energy is considered to be a marker of successful energy delivery, and resultant non-inducibility of the tachycardia is accepted as a successful endpoint for RFCA (1, 2).
However, for patients with non-inducible ventricular tachycardia (VT) or patients in whom the re-induction of VT is difficult due to catheter-induced mechanical trauma, it is impossible to deliver RF energy during on-going VT. Furthermore, the evaluation of procedural success and the recurrence of ILVT can be problematic.
In the practice of radiofrequency ablation for certain kinds of arrhythmias, the rhythm responses during energy delivery, such as junctional beats for atrioventricular nodal re-entrant tachycardia (AVNRT) and transient acceleration of the rate for right ventricular outflow tract tachycardia (RVOT VT), are regarded as indirect markers of effective energy delivery (3-5). However, there are no reports describing the rhythm response during RF delivery for ILVT, especially with respect to energy delivery during sinus rhythm.
We have observed the ventricular rhythm changes during the delivery of RF energy in patients with ILVT. Herein we describe the significance of thermal response that shows repetitive ventricular response exhibiting similar morphology to clinical VT.
The study subjects consisted of 11 patients with ILVT (10 males:1 female, age 31.6±8.3 yr) in whom RFCA was attempted during sinus rhythm, because of either repeated mechanical interruption of tachycardia while manipulating the catheter or difficulty of re-induction of tachycardia. The cases consisted of the patients admitted to the 3 participating hospitals (Gangnam St. Mary's Hospital, Uijeongbu St. Mary's Hospital and Incheon St. Mary's Hospital) between January 2004 and January 2007.
Clinical tachycardia was documented by a 12-lead surface electrocardiogram in all patients. The VTs showed typical right bundle branch block morphology with superior axis deviation. All patients had structurally normal hearts. Our Institutional Review Board approved this study (IRB No. UC09RAS10084). Informed consent was obtained from all patients.
After anti-arrhythmic drugs were withdrawn for at least five half-lives, all patients were placed under electrophysiological evaluation. Standard 6-Fr catheters were introduced into the right ventricular apex (RVA) and/or the right ventricular outflow tract (RVOT), and into the His bundle region via the femoral veins. Programmed stimulation was employed in an effort to induce sustained VT with a right bundle branch block morphology and a left axis deviation (Fig. 1).
The stimulation protocol consisted of programmed ventricular stimulation from the RVA and/or the RVOT at two drive cycle lengths with up to two extra stimuli and incremental pacing up to 250 ms. When sustained ILVT was not induced during the baseline state, the stimulation was repeated after isoproterenol infusion (1 to 3 µg/min).
The ablation catheter was placed in the left ventricle crossing the aortic valve retrogradely. After the induction of ventricular tachycardia, left ventricular mapping was performed using a steerable catheter with a 4-mm electrode tip (Navi Star, Biosense Webster Inc., Diamond Bar, CA, USA). Bipolar electrograms were recorded for an intracardiac electrogram using EA mapping (Prucka CardioLab 7000, GE Healthcare, Waukesha, WI, USA) and with the bandpass filter set at 10-400 Hz.
This study subjects whose VTs were repeatedly terminated by catheter manipulation and/or who were difficult to reinduce by programmed stimulation. Therefore, RF energy was applied during sinus rhythm in these subjects.
The attempted site of RF ablation was selected as the area in which the tachycardia was presumed to be mechanically terminated with catheter manipulation and which showed Purkinje-potential (P-potential). In cases of difficulty of VT re-induction, we searched for sites with P-potential (Fig. 2) and attempted to start ablation from the site closest to the apical septum.
We focused on the rhythm change of the repetitive ventricular responses (>3 consecutive QRS beats) during RF application, especially showing similar morphology to clinical VT.
Unless a repetitive ventricular response was noted within 15 sec, the energy delivery was terminated. However, once the repetitive ventricular response showing similar morphology to clinical VT appeared, the RF energy delivery was tried to be continuing for 60 sec from the distal electrode of the mapping catheter with a pre-selected temperature of 60℃. After each RF pulse, re-induction of ILVT was tried with programmed stimulation.
The patterns and clinical implications of the repetitive ventricular response were evaluated with respect to the results of RFCA and the clinical course during the follow-up period.
The pattern of ventricular rhythm response during RF energy delivery varied according to the attempted site of ablation, including no rhythm change, repetitive ventricular premature contractions (VPCs), non-sustained VT (Fig. 3), and sustained VT (Fig. 4). One case exhibited ventricular fibrillation (VF) during RF energy delivery.
Although a repetitive ventricular response did not occur with every RF application, repetitive ventricular responses were usually repeated or sustained during the period of RF current application. With the exception of the one patient who had an occurrence of ventricular fibrillation, repetitive ventricular responses always disappeared immediately after cessation of energy delivery in all patients.
Analysis of 12-lead ECG patterns revealed that the QRS morphology of the repetitive ventricular response varied from a VPC of different morphology to VTs almost identical to clinical VT (Fig. 5). The repetitive ventricular response that was almost identical to clinical VT was noted more than once in 10 out of 11 patients (Table 1).
The mean number of RF applications was 11.1 (±6.5)/patient (Table 1). Two patients received second procedures due to the recurrence of ILVT, one at three days after the initial procedure and another, a year later. In the case of the former, no repetitive ventricular response was observed during his first procedure, but it had been regarded as successful procedure because there was no re-induction of VT. The mean follow-up period for all patients was 36.2±12.8 months. The clinical course of the remaining patients with repetitive ventricular responses was favorable without recurrence (Table 1).
RFCA could be considered as a potential first-line therapy for patients with ILVT because those VT can be eliminated by ablation in a high percentage of patients. In the practice of RFCA for ILVT, the termination of VT with RF energy delivery is considered a marker of successful energy delivery (1, 2). However, it is sometimes impossible to deliver RF energy during on-going VT, especially in patients with mechanical interruption of VT resulting from catheter-related mechanical trauma or with non-inducible VT. In those patients, the evaluation of procedural success and ILVT recurrences can be problematic.
In the present study, special focus was given to the ventricular rhythm response that was noted with application of RF energy. The ventricular response triggered by RF current application was variable with the responses ranging from no rhythm change to repeated VPCs, non-sustained VTs or sustained VTs, and the morphology was also variable. However, the repetitive ventricular responses which we defined as more than 3 consecutive ventricular beats and also having a similar morphology to clinical VT were observed in most of the study subjects.
A similar response has been previously reported with RF ablation for AVNRT or automatic atrial tachycardia. This has also been observed, although rarely, in ablation for an accessory pathway, atrial flutter, or VT without underlying heart diseases (6, 7). The occurrence of junctional beats during slow pathway ablation for AVNRT could be an example. Accelerated junctional tachycardia is accepted as a sensitive marker of successful ablation in cases of RF ablation of slow pathway potential in AVNRT (8). In my opinion though, there have been no such reports regarding the ILVT.
Successful ablation of ILVT can be performed at the site of the earliest Purkinje potential. However, these critical potentials have a limitation for evaluating procedural success, especially for the patients who exhibit trouble with inducing the tachycardia repeatedly. We performed this study for the evaluation of the significance of repetitive ventricular response in patients with ILVT. And the successful clinical course of the patients in this study could be a evidence that repetitive ventricular response during RF ablation is a successful procedural end point rather than an epiphenomenon of the ablation.
The differences in electrophysiologic cellular characteristics between ablation sites with or without the repetitive ventricular response during RF applications were not evaluated in this study. However, these responses suggest that myocardium having an automaticity could be sensitive to heat. In experimental studies, hyperthermia caused a progressive depolarization of membrane potential, significant change in the action potential (AP) characterized by a temperature-dependent shortening of action potential duration (APD) and a decrease in the maximum rate of rise of the AP, and the development of abnormal automaticity. These electrophysiologic changes can be considered as mechanisms of RF current-induced tachyarrhythmia (9-13).
The underlying mechanism of ILVT is widely accepted as a reentry through the left ventricular Purkinje network (14-16) and the cells comprising the Purkinje fibers have the characteristic of automaticity. Therefore it seems plausible that the Purkinje cells involved in re-entry of ILVT might exhibit similar responses when exposed to heat by RF energy.
The repetitive ventricular responses varied by patient in their patterns, but were characterized by repetition or continuation throughout the whole period of the RF current applications in most of the patients. In case where the slow conduction zone of the reentry circuit is wide, the ablation of the entrance site of the circuit showed different QRS configuration (17).
One study showed seven patients among 27 patients achieved successful ablation of tachycardia, and the pace map 12-lead ECG at site of successful ablation achieved was exhibited a different QRS complex configuration from that seen during the tachycardia (17). However, all the patients in this study showed the QRS morphology of repetitive ventricular responses similar to the clinically documented tachycardia more than once during the ablation and we thought this response could be an evidence of energy delivery on the critical site adjacent to exit site. We did not count the exact the number of appearance of repetitive ventricular responses with different morphologies. It should be noted the repetitive ventricular responses with different morphologies were not rare. But, we thought the repetitive ventricular responses with similar morphology with clinical tachycardia are clinically relevant.
Nakagawa et al. first reported the importance of P-potential in ablation for ILVT (18), while Tsuchiya et al. emphasized the significance of late diastolic potential and pre-systolic potentials in the VT circuit (19). Even though the successful ablation sites were different in these two research groups, the findings of these studies strongly suggest that the P-potential is an important marker of the critical site in LV tachycardia. On the other hand, these P-potentials were found over a relatively wide area, even where they were not actually incorporated as critical components of VT circuits. This suggests that the value of the P-potential could be more limited (20, 21). However, despite of inherent limitation of P-potentials, the fact that the QRS morphology of the repetitive ventricular response noted in most of this study subjects was almost identical to that of clinical VT could indicate that the repetitive ventricular response occurred from a site close to the origin of the VT.
There were case reports that successful ablation of idiopathic left ventricular tachycardia was achieved at site away from the tachycardia exit site, and even a site over the superior midleft ventricular septum, away from the putative tachycardia exit site (17). But, in our cases, the successful sites were in the tachycardia exit site with a mean PP-QRS interval of 21.2 ms. It is longer than that of the previous study (17) and we believed there was no correlation between the PP-QRS interval and repetitive ventricular responses.
Recently study reported a case of idiopathic right ventricular tachycardia was repeated in which VF was triggered by a delivery of RF energy (22). Stimulation from temperature elevation and/or mechanical stress during the RF energy delivery may cause VF in patients with idiopathic right ventricular arrhythmia. However, in the present study, we experienced only one case of VF during ablation, and we were uncertain whether this VF is induced by a delivery of RF energy or not.
Catheter-induced mechanical artifact seemed less likely to be the mechanism of repetitive ventricular response, because the repetitive ventricular response was observed only during the period of RF current application. Thus, this repetitive ventricular response could be explained by thermal response which is induced by ablating myocardium.
Thermal effect can enhance abnormal automatic activity. Repetitive ventricular response has been observed in RF ablation for AVNRT and automatic atrial tachycardia. Meanwhile, there was no clear explanation regarding the cycle length of the repetitive ventricular responses and there was even a report that fast junctional tachycardia could be an impending AV block rather than a meaningful energy delivery for AVNRT (23). Considering this we thought the cycle length of repetitive ventricular responses could be variable.
Iakobishvili et al. (24) reported the cycle length of accelerated junctional rhythm during RF ablation of slow pathway was variable, and Chinushi et al. (7) reported that the appearance followed by a slowing and/or disappearance of repetitive ventricular responses during RF application was consistent with successful RF ablation in RVOT VT. We experienced the similar responses in our cases, so a slowing and/or disappearance of repetitive ventricular response during RF application might be another marker of successful ablation, but we need further studies with a larger number of patients.
The area heated by a standard 4 mm tip ablation catheter is known to be confined within a 5 mm radius. Considering the limited area affected by RF induced-heat, this response which we herein term "thermal mapping" could provide better accuracy than pace mapping for selection of ablation site.
Many of the previously described mapping techniques are depending on inducibility of sustained VT. In that approach, targeting presystolic P-potential is useful in treating ILVT. However, in cases of non-inducible ILVT or having trouble with repeated induction, it has limitation to assess the efficacy of the procedure. There are a few reports that ablation of ILVT was successful during sinus rhythm in patients with non-inducible or non-stable ILVT, but the number of the study subject was very small (25, 26).
In this study, we have followed up the patients for relatively long period. Most importantly, based on the favorable clinical outcomes of this study's subjects, we conclude that "thermal mapping" reflected by repeated ventricular responses is useful for guiding RF ablation for ILVT, particularly in the circumstances of difficult VT induction.
The most significant limitation of the "thermal mapping" approach is the increase in the number of RF current applications which must be applied in a somewhat blind fashion simply to target the P-potential. Nevertheless, we believe that the approach may be valuable for patients who otherwise would leave the catheterization lab without any treatment.
The number of patients in this study was small. Further studies with a larger number of patients and including basic experiments elucidating the heat effect on the myocardium will provide more precise and meaningful results.
In conclusion, in the practice of RF ablation for ILVT, the occurrence of a repetitive ventricular response triggered by RF current application, most specially a response with similar morphology to clinical VT, is an indirect marker of effective energy delivery to an appropriate site, at least for patients in whom the delivery of RF energy during on-going VT is not possible.
Figures and Tables
 | Fig. 1Twelve-lead surface electrocardiograms of induced ventricular tachycardia for patients No. 4 and No. 10, showing RBBB and superior axis.
RBBB, Right bundle branch block.
|
 | Fig. 2An example of local electrocardiogram from an ablation catheter shows both the P-potential (↓) and sinus rhythm changes to wide QRS tachycardia with radiofrequency energy delivery.
P-potential, Purkinje-potential.
|
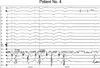 | Fig. 3During the repetitive ventricular response which occurred during radiofrequency energy delivery in patient No. 4, the P-potentials (↓) preceding wide QRS were noted. These repetitive ventricular responses were repeated during radiofrequency energy delivery.
P-potential, Purkinje-potential.
|
 | Fig. 4The eletrocardiograms from patients No. 4 and No. 10 showing sudden rhythm change with the start of radiofrequency energy delivery (arrow). |
References
1. Page RL, Shenasa H, Evans JJ, Sorrentino RA, Wharton JM, Prystowsky EN. Radiofrequency catheter ablation of idiopathic recurrent ventricular tachycardia with right bundle branch block, left axis morphology. Pacing Clin Electrophysiol. 1993. 16:327–336.

2. Coggins DL, Lee RJ, Sweeney J, Chein WW, Van Hare G, Epstein L, Gonzalez R, Griffin JC, Lesh MD, Scheinman MM. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994. 23:1333–1341.
3. Lee MA, Morady F, Kadish A, Schamp DJ, Chin MC, Scheinman MM, Griffin JC, Lesh MD, Pederson D, Goldberger J, Calkins H, deBuitleir M, Kou WH, Rosenheck S, Sousa J, Langberg JJ. Catheter modification of the atrioventricular junction with radiofrequency energy for control of atrioventricular nodal reentry tachycardia. Circulation. 1991. 83:827–835.

4. Jackman WM, Beckman KJ, McClelland JH, Wang X, Friday KJ, Roman CA, Moulton KP, Twidale N, Hazlitt HA, Prior MI, Margolis PD, Calame JD, Overholt KD, Lazzara R. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow pathway conduction. N Engl J Med. 1992. 327:313–318.
5. Clyne CA, Athar H, Shah A, Kahr R, Rentas A. Thermal mapping of right ventricular outflow tract tachycardia. Pacing Clin Electrophysiol. 2007. 30:343–351.

6. Jentzer JH, Goyal R, Williamson BD, Man KC, Niebauer M, Daoud E, Strickberger SA, Hummel JD, Morady F. Analysis of junctional ectopy during radiofrequency ablation of the slow pathway in patients with atrioventricular nodal reentrant tachycardia. Circulation. 1994. 90:2820–2826.

7. Chinushi M, Aizawa Y, Ohhira K, Fujita S, Shiba M, Niwano S, Furushima H. Repetitive ventricular responses induced by radiofrequency ablation for idiopathic ventricular tachycardia originating from the outflow tract of the right ventricle. Pacing Clin Electrophysiol. 1998. 21:669–678.

8. Li HG, Klein GJ, Stites HW, Zardini M, Morillo CA, Thakur RK, Yee R. Elimination of slow pathway conduction: an accurate indicator of clinical success after radiofrequency atrioventricular node modification. J Am Coll Cardiol. 1993. 22:1849–1853.

9. Nath S, Haines DE. Biophysics and pathology of catheter energy delivery systems. Prog Cardiovsc Dis. 1995. 37:185–204.

10. Cote JM, Epstein MR, Triedman JK, Walsh EP, Saul JP. Low-temperature mapping predicts site of successful ablation while minimizing myocardial damage. Circulation. 1996. 94:253–257.

11. Nath S, Lynch C 3rd, Whayne JG, Haines DE. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implantations for catheter ablation. Circulation. 1993. 88:1826–1831.
12. Chinushi M, Aizawa Y, Ohhira K, Fujita S, Shiba M, Niwano S, Furushima H. Repetitive ventricular responses induced by radiofrequency ablation for idiopathic ventricular tachycardia originating from the outflow tract of the right ventricle. Pacing Clin Electrophysiol. 1998. 21:669–678.

13. Yi KH, Han HS. Cellular electrophysiology of fast pathway ablation of rabbit atrioventricular node. J Korean Med Sci. 2000. 15:494–500.

14. Tomokuni A, Igawa O, Yamanouchi Y, Adachi M, Suga T, Yano A, Miake J, Inoue Y, Fujita S, Hisatome I, Shigemasa C. Idiopathic left ventricular tachycardia with block between Purkinje potential and ventricular myocardium. Pacing Clin Electrophysiol. 1998. 21:1824–1827.

15. Kottkamp H, Hindricks G, Willems S, Haverkamp W, Wichter T, Breithardt G, Borggrefe M. Idiopathic left ventricular tachycardia: New insights into electrophysiological characteristics and radiofrequency catheter ablation. Pacing Clin Electrophysiol. 1995. 18:1285–1297.

16. Tada H, Nogami A, Naito S, Tomita T, Oshima S, Taniguchi K, Aonuma K, Iesaka Y. Retrograde Purkinje potential activation during sinus rhythm following catheter ablation of idiopathic left ventricular tachycardia. J Cardiovasc Electrophysiol. 1998. 9:1218–1224.

17. Wen MS, Yeh SJ, Wang CC, Lin FC, Wu D. Successful radiofrequency ablation of idiopathic left ventricular tachycardia at a site away from the tachycardia exit. J Am Coll Cardiol. 1997. 30:1024–1031.

18. Nakagawa H, Beckman KJ, McClelland JH, Wang X, Arruda M, Santoro I, Hazlitt HA, Abdalla I, Singh A, Gossinger H, Sweidan R, Hirao K, Widman L, Pitha JV, Lazzara R, Jackman WM. Radiofrequency catheter ablation of idiopathic left ventricular tachycardia guided by a Purkinje potential. Circulation. 1993. 88:2607–2617.

19. Tsuchiya T, Okumura K, Honda T, Honda T, Iwasa A, Yasue H, Tabuchi T. Significance of late diastolic potential in verapamil-sensitive idiopathic left ventricular tachycardia. Circulation. 1999. 99:2408–2413.
20. Wen MS, Yeh SJ, Wang CC, Lin FC, Wu D. Successful radiofrequency ablation of idiopathic left ventricular tachycardia at a site away from the tachycardia exit. J Am Coll Cardiol. 1997. 39:1024–1031.

21. Aizawa Y, Chinushi M, Kitazawa H, Washizuka T, Takahashi K, Shiba M, Ohhira K, Abe A, Shibata A. Spatial orientation of the reentrant circuit of idiopathic left ventricular tachycardia. Am J Cardiol. 1995. 76:316–319.

22. Ito S, Tada H, Lee JD, Miyamori I. Ventricular fibrillation induced by a radiofrequency energy delivery for idiopathic right ventricular outflow tachycardia. Int J Cardiol. 2008. 128:e65–e67.

23. Lipscomb KJ, Zaidi AM, Fitzpatrick AP, Lefroy D. Slow pathway modification for atrioventricular node re-entrant tachycardia: fast junctional tachycardia predicts adverse prognosis. Heart. 2001. 85:44–47.

24. Iakobishvili Z, Kusniec J, Shohat-Zabarsky R, Mazur A, Battler A, Strasberg B. Junctional rhythm quantity and duration during slow pathway radiofrequency ablation in patients with atrioventricular nodal re-entry supraventricular tachycardia. Europace. 2006. 8:588–591.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download