This article has been corrected. See "Erratum: Correction of Belonging Institution of One Author" in Volume 25 on page 1258.
Abstract
Despite remarkable progress in understanding and treating gastrointestinal stromal tumors (GISTs) during the past two decades, the pathological characteristics of GISTs have not been made clear yet. Furthermore, concrete diagnostic criteria of malignant GISTs are still uncertain. We collected pathology reports of 1,227 GISTs from 38 hospitals in Korea between 2003 and 2004 and evaluated the efficacy of the NIH and AFIP classification schemes as well as the prognostic factors among pathologic findings. The incidence of GISTs in Korea is about 1.6 to 2.2 patients per 100,000. Extra-gastrointestinal GISTs (10.1%) are more common in Korea than in Western countries. In univariate analysis, gender, age, tumor location, size, mitosis, tumor necrosis, vascular and mucosal invasions, histologic type, CD34 and s-100 protein expression, and classifications by the NIH and AFIP criteria were found to be significantly correlated with patient's survival. However, the primary tumor location, stage and classification of the AFIP criteria were prognostically significant in predicting patient's survival in multivariate analysis. The GIST classification based on original tumor location, size, and mitosis is more efficient than the NIH criteria in predicting patient's survival, but the mechanism still needs to be clarified through future studies.
Since Schaldenbrand and Appelman applied the term "stromal tumor" to collectively refer to a group of mesenchymal neoplasms of gastrointestinal tract in 1984 (1), there was considerable confusion with regards to the classification of the tumors. However, during the past two decades this confusion was eliminated, and clarification and restructuring became reliable with the introduction of the KIT protein expression in gastrointestinal stromal tumors (GISTs). Because most GISTs have an activating mutation in the c-kit proto-oncogene that leads to expression of KIT protein, the immunohistochemical stain for the KIT (CD117) is now used by pathologists to distinguish GISTs from non-GISTs mesenchymal tumors in gastrointestinal tract (2, 3). This was the chief reason why epidemiologic studies of GISTs in Korea were unreliable before the 2000s. Based on the biological characteristics of this tumor, treatment with KIT/PDGFRα tyrosine kinase inhibitors, such as imatinib, can lead to complete or partial remission when the tumor is unresectable or in a metastatic setting (4). Therefore, accurate pathological diagnosis is mandatory for proper treatment of patients with mesenchymal tumors of gastrointestinal tract. Recently, protein kinase C theta (PKC-θ) (5) and DOG1 (6) over-expression in addition to mutation analysis of the c-kit and PDGFRα (7) were described as diagnostic markers for GISTs.
Despite remarkable progress in understanding and treating GISTs, pathologists still have a difficulty in classifying GISTs because of a lack of concrete diagnostic evidence to predict patient's prognosis. The incidence of GIST varies as reported in different studies from different countries (8-11). Gender, race, and location distribution of GISTs are also uncertain. Furthermore, there is no agreement on prognostic factors of GISTs. In 2002, National Institute of Health (NIH) reported a consensus approach in diagnosing GISTs (2). The efficacy of the NIH criteria had been discussed, although it is commonly used in clinical setting. Recently, new diagnostic criteria in predicting prognosis of GISTs have been proposed by Miettinen et al in the Armed Forces Institutes of Pathology (AFIP) (12, 13). It has caused some confusion with regards to the terminology used in cancer registration, and it became difficult to identify the actual incidence of malignant GISTs.
To create informative and standardized pathological reports of GIST, we need to evaluate contents of pathology reports collected nationwide and to identify significant factors that predict the prognosis. We previously analyzed pathological characteristics of GISTs and described the pitfalls in interpretation of KIT expression as well as pathological diagnosis of GISTs (14) through a nationwide study of GISTs in Korea from 2001 to 2002 (14). One outstanding phenomena found in previous research was that small intestinal and esophageal GISTs were more common in Korea than in Western countries, but the incidence and prognostic factors of GISTs in Korea have not been made clear yet.
In this study, we firstly described the population based incidence of GIST in Korea. Then, we identified pathological characteristics that should be mentioned in pathology reports of GISTs to predict patient's prognosis and evaluated the efficacy of the NIH and AFIP classification criteria. Data obtained from this nationwide multi-institutional study may provide an insight in the epidemiological characteristics and prognostic stratification of GISTs in Korea.
To identify the population based incidence and trends in epidemiology of malignant GISTs in Korea, we reviewed all data collected by the Korean National Cancer Institute (NCI) and correlated the data with the population information of the Korea National Statistical Office. All tumors in the NCI Registry were identified by the International Classification of Disease for Oncology (ICD-O), 3rd edition, from the World Health Organization (WHO).
For survival analysis, we retrospectively collected nationwide multi-institutional data of pathology reports diagnosed as GISTs not only in gastrointestinal tract but also in extragastrointestinal areas from 2003 to 2004.
The list of participant hospitals were as follows: Sungkyunkwan University Seoul Samsung Medical Center and Kangbuk Samsung Medical Center, Asan Medical Center, Seoul National University Hospital and Bundang Hospital and Boramae Hospital, Yonsei University Severance Hospital and Kangnam Severance Hospital and Wonju Christian Hospital, Chonnam University Hospital, Ewha Woman's University Hospital, Ajou University Hospital, Yeungnam University Hospital, Inje University Seoul Paik Hospital, Busan Paik Hospital and Ilsan Paik Hospital, The Catholic University of Korea Seoul St. Mary's Hospital, Uijeongbu St. Mary's Hospital and St. Vincent Hospital, National Cancer Center, Dong-A University Hospital, Korea University Seoul Hospital and Ansan Hospital, Choongnam University Hospital, Daegu Catholic University Hospital, Soonchunhyang University Seoul Hospital and Bucheon Hospital, Inha University Hospital, Busan University Hospital, Choongbuk University Hospital, Eulji University Hospital, Chonbuk University Hospital, Gosin University Hospital, Hallym University Hospital, Daegu Fatima Hospital, Kyunghee University Hospital, CHA Medical School Hospital, and Busan Baptist Hospital. Institutional Review Board of Sungkyunkwan University Kangbuk Samsung Medical Center gave a permission for this research (C 0847).
We collected data of age, gender, primary tumor location, tumor size, mitotic count, diagnosis, stage, and immunohistochemical findings (c-kit, desmin, actin, s-100, CD34) in patients, if they were described. Then, we evaluated the contents of pathology reports. GIST locations were categorized into esophagus, stomach, small intestine, large intestine, and extra-gastrointestinal areas. Follow-up data were taken from the Korea National Statistical Office. All the data were employed to perform an anonymous and aggregate statistical analysis.
To estimate prognostic significance of the classification schemes of GISTs, each tumor was re-classified the basis of descriptions in the pathology reports. The used diagnostic criteria were those proposed by the National Institutes of Health (NIH) GIST Workshop (2001) and the AFIP (2002). The NIH criteria includes four risk groups; very low risk (<2 cm and <5 mitoses/50 HPF), low risk (2-5 cm and <5 mitoses/50 HPF), intermediate risk (<5 cm and 6-10 mitoses/50 HPF or 5-10 cm and <5 mitoses/50 HPF), and high risk (>5 cm and >5 mitoses/50 HPF or >10 cm regardless of mitotic activity or >10 mitoses/50 HPF regardless of the tumor size). On the other hand, the AFIP criteria (2002) includes three groups; benign (stomach ≤5 cm and <5/50 HPF; intestine ≤2 cm and <5/50 HPF), uncertain or low malignant potential (stomach >5 cm and ≤10 cm and <5/50 HPF; intestine >2 cm but ≤5 cm and <5/50 HPF), and malignant (stomach >10 cm or ≥5/50 HPF; intestine >5 cm or ≥5/50 HPF).
Data were presented as numbers (%) for categorical variables. To estimate the association between eligible variables and mean survival time, the Kaplan-Meier test was applied together with the log-rank test to compare various groups. We conducted the Cox proportional hazard regression analysis to estimate hazard risk ratios and 95% confidence intervals (CI) of the possible risk factors for survival after adjustment in age and sex. The SPSS (version 13.0) was used for statistical analyses. A P value of <0.05 was considered statistically significant.
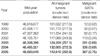
There were 1,233 cases of malignant GISTs in the National Cancer Institute (NCI) Registry from 1999 to 2005 (Table 1). Table 1 shows the incidence of malignant GISTs in comparison with all malignant tumors registered. There was a big shift in GISTs incidences between 1999 and 2002 and also from 2003 to 2005, while the incidence of all malignant tumors was almost the same during the same time. Extra-gastrointestinal GISTs (8.3-8.7%) showed to be more common than esophageal GISTs (0.7-1.1%).
We collected 1,227 pathology reports from the 38 participating hospitals from January 1, 2003 to December 31, 2004. The characteristics of GISTs and contents of pathology reports collected nationwide are shown in Table 2. The range of patient age was from 11 to 86 yr old (mean 57.83±12.62); 5 (0.4%) was in their 1st decade, 20 (1.6%) in 2nd, 87 (7.1%) in 3rd, 200 (16.3%) in 4th, 302 (24.6%) in 5th, 382 (31.1%) in 6th, 210 (17.1%) in 7th, and 21 (1.7%) in 8th. Male to female ratio was 1 to 1.7. The most common location of tumor was stomach. Extra-gastrointestinal locations were omentum and mesentery (45.1%). Then, pelvis (9.8%), intra-abdominal (34.3%), retroperitoneum (3.9%), abdominal wall (3.9%), and pancreas (3%) were found to be occurring sites of extra-gastrointestinal GISTs. Liver (46), lymph node (9), bone, lung, spleen, diaphragm, and so on were found to be metastatic sites of GISTs. One hundred cases (8.2%: 3 in the esophagus, 85 in the stomach, 8 in the small intestine, 1 in the large intestine and 1 in extra-gastrointestinal) were incidentally found during operations for other diseases. More than 90% of the tumors showed the result of c-kit immunostain in pathology reports in contrast with CD34, actin and s-100 protein in which about 80% were found to have. Almost all GISTs, which provided the results of immunostains, were c-kit positive (96.2%). The relationship between tumor location and malignancy, defined by variable criteria are presented in Tables 3, 4, 5.
The incidence of gastric GIST was slightly increased in ages older than 60 yr, in contrast to decreases in the incidence of the small intestinal GISTs. However, the location of tumor was not significantly related with patient's age (P=0.083). In general, it showed male preponderance. Esophageal, large intestinal, and extra-gastrointestinal GISTs were more common in males than in females, but gastric GISTs were more common in females (P=0.027).
In the collected pathology reports, GISTs were classified by the NIH criteria (55.4%), the AFIP criteria (41.7%), and other (2.9%). To compare the prognostic value of the diagnostic criteria, we re-classified each GIST by the NIH criteria as well as the AFIP criteria based on the descriptions in the pathology reports, including tumor, size, location, and mitosis (Tables 3, 4). About half of the GISTs in our study were malignant according to the AFIP criteria. Benign tumors were common in stomach in contrast to malignant tumors which were more common in intestinal and extra-gastrointestinal areas (P=0.000). High risk tumors, as classified by the NIH criteria, were also more common in the small and large intestinal and extra-gastrointestinal locations than in stomach (P=0.001). Most of extra-gastrointestinal tumors were malignant (88.6%) or high risk (72.1%). We also reclassified GISTs into eight groups based on tumor size and mitosis according to a new classification by Miettinen and Lastosa 2006 (15) (Table 5). However, statistical analysis was not available due to the small number of tumors in some categories. As shown in Table 5, tumor size was correlated with mitosis. In the group with mitotically active GISTs, gastric tumors tend to be small in size compared with tumors in intestine, extra-gastrointestinal areas, and esophagus. The description of tumor necrosis, vessel and mucosal invasions, and histologic type were found only in 21.5%, 8.5%, 11.8%, and 65.6% of pathology reports collected for this study, respectively.
During the follow-up study period, we found that 102 patients died as a result of GISTs. Most GISTs, which caused mortality, were malignant (80.4%) or high risk (66.7%). However, 1.8% of benign or uncertain malignant tumors and 6.9% of very low risk, low risk or intermediate tumors led to mortality. In the same risk group by the NIH criteria, the incidence of patients, who died of the diseases, was higher in small intestinal and extra-gastrointestinal GISTs than gastric ones. The malignant GISTs by the AFIP criteria also demonstrated a higher mortality rate.
All the variables except for smooth muscle actin immunoexpression that was significantly correlated with the survival were analyzed in this study with univariate analysis (Figs. 1, 2, 3, 4). In ages older than 60 yr, male gender, small intestinal and extra-gastrointestinal location, larger than 10 cm in size, and mitosis more than 5/50 HPF pointed to poor survival rate. All histopathological variables analyzed in the study: mucosal and vessel invasions and necrosis were significantly related with the survival (Fig. 2, P=0.000, P=0.000, and P=0.000, respectively). Spindle cell type tumors showed a better prognosis than mixed, epithelioid, and pleomorphic cell types (Fig. 2, P=0.016). In the classification of GISTs by the NIH criteria, there was no prognostically significant difference between very low and low risk versus intermediate risk tumors, but high-risk tumors pointed significantly to a shorter survival time (Fig. 4, P=0.000). According to the AFIP criteria, the median survival time of malignant tumors was significantly shorter than those of benign and uncertain malignancy (Fig. 4, P=0.000).
In multivariate analysis, tumor location, patient's age, malignant GISTs by the AFIP criteria, and stages of tumors showed significant correlation with the prognosis (Table 6). People with tumor sizes more than 2 cm were more likely at an increased risk of death compared to those with tumor sizes less than 2 cm, but this data was statistically not significant (P=0.247). In terms of tumor location, the risk was 1.985 and 2.423 times higher in small intestines and extra-gastrointestinal than in stomachs (P=0.030 & 0.014), but the risk in large intestinal GISTs was not higher (P=0.602). Malignant GISTs by the AFIP criteria revealed a 6.211 times higher risk of mortality compared with benign and borderline ones (P=0.027). The risk in aggressive behavior of GISTs by the NIH criteria slightly increased in intermediate tumors compared with very low and low risk GISTs (odds ratio=0.443 & 0.585), but this data was not statistically significant (P=0.524). GISTs limited to organs revealed better survival of patients than tumors, which invaded adjacent organs or metastasis.
The immunoexpression of α-smooth muscle actin, CD34 and s-100 protein, necrosis, and mucosal and vascular invasions were not available for multivariate analysis because of the small number of results.
As a result of this study, the GIST classifications based on original tumor location together with size and mitosis is more efficient than the NIH criteria in predicting patient's survival. However, the mechanism still needs to be clarified through future studies.
This is the first description of a population based on the incidence of GISTs in Korea. In the review of the Korean NCI Registry, we found a huge change regarding the incidences of malignant GISTs between 1999-2002 and 2003-2005. For example, the incidence of malignant GISTs in 2003 (0.69 per 100,000 population) was a double that of 2002 (0.24 per 100,000), even though there were no significant changes in the incidences of all malignant tumors during the same time. In regard with the change in incidences of GISTs, several historical backgrounds may be related with them. The main reasons of the incidence increase of GISTs during 2002-2005 in Korea is related to an improved understanding of pathobiology, the treatment of GISTs, the introduction of schematic diagnostic criteria proposed by the NIH consensus workshop together with improved convenience of immunohistochemical staining for c-KIT. Changes in the GIST incidences similar to those in our study also has been described by Goettsch et al. (16) and Steigen and Eide (17).
Since the NIH publication, which was unfortunately based on a consensus opinion rather than actual follow-up data (2), numerous additional pathologic and biologic variables have been evaluated as prognostic factors. In 2002 Miettien et al. (18) analyzed a large AFIP series of GISTs coupled with long term follow-up data and then proposed guidelines for the evaluation of GISTs malignancies. Taking the Korean NCI Registry in consideration, and including only tumors coded as having malignant behavior, the incidence of all GISTs in Korea can be estimated as 1.6 per 100,000 by the AFIP criteria. The proportion of malignant GISTs by the AFIP criteria was 43.1%. However, by the NIH criteria, the proportion of high-risk GISTs was 32.1%, and the incidence of GISTs was estimated 2.2 per 100,000. The incidence of GISTs have been previously reported at 1-2 per 100,000 population by Miettinen and Lastosa (3), 1.85-2.2 per 100,000 population in the United States by Fletcher et al. (2), 2 per 100,000 population in southwestern Sweden by Nilsson et al. (9), and 1.1 per 100,000 population in Iceland by Tryggvason et al. (10). The incidence of GIST in Korea did not appear to be different from theirs.
In the review of pathology reports, we found that most hospitals in Korea used either the NIH criteria or the AFIP criteria to predict the prognosis of GISTs. The results of our study, different diagnostic criteria and terminology in two diagnostic schemes, may have created some confusion in classifying and estimating the incidence of GISTs. However, the identification of GISTs with poor prognosis is very important in the choice of treatment modality to improve the survival. We found a prognostic significance of the AFIP criteria in univariate as well as multivariate analyses. In our previous report on guideline for cancer registration, the intermediate and high risk tumors were regarded as malignancy. However, the guideline is based on a consensus opinion rather than an actual follow-up data (19). In this study, the prognosis of intermediate risk groups of GIST by the NIH criteria was not different from those of the very low and low risk tumor groups when follow-up data were examined. Previously published reports also described no prognostic differences in intermediate risk GISTs and the very low and low risk ones (20). In multivariate analysis of our study, the patients with intermediate and high risk GISTs were more likely to increase the risk of mortality compared with very low and low risk GISTs, but it was statistically not significant (P=0.325 and 0.548, respectively). It appeared to be appropriate that only high-risk tumors were regarded as malignant GISTs. However, a few patients with low and intermediate risk tumors died of diseases in our study. The prognosis of low and intermediate risk GISTs by the NIH criteria remains to be clarified through long term follow-up. The prognostic efficacy of the NIH criteria has been discussed by several researchers (9, 21), and the modification of the NIH criteria (20-22) was published recently to improve the risk-based stratification of GISTs. The original NIH criteria adopted only tumor size and mitosis but the significance of tumor size and location was considered on AFIP criteria (12, 18). The impact of the location of GISTs on the patient's survival is still controversial. Some investigators demonstrated a more aggressive behavior in intestinal GISTs than in gastric GISTs (9-12, 21, 23, 24), while others found no differences in the patient's survival (25). In the results of our study, the AFIP criteria as well as the original tumor location were prognostically significant in multivariate analysis. There seems to be several explanations for prognostic significances of the tumor location. Firstly, the prognostic significance of the tumor location may be related to early detection of gastric GISTs. This is supported by the fact that gastric GISTs were significantly smaller than the non-gastric ones (10). The results of our study demonstrated that the malignant GISTs by the AFIP criteria or high risk by the NIH criteria were far more common in the intestine and extra-gastrointestinal than in the stomach at the time of diagnosis. Secondly, Miettinen et al. recently reported the true biologic differences between gastric and non-gastric GISTs (26). The mutation in exon 9 of the c-kit gene, which is related to more aggressive tumors, was reported to be more frequently found in small intestinal GISTs (24). Although Park et al. (25) described that the tumor location was significant to predict the overall survival of patients in univariate analysis of 93 high-risk GISTs, but c-kit exon 9 mutations were not related with poor prognosis in Korean patients. In the data of patients, who died of the disease in our study, we found that the proportion of intestinal and extra-gastrointestinal GISTs were higher than gastric GISTs among the same categories by the NIH criteria. This means that the anatomical tumor location has to be related to a patient's survival but it remains to be clarified as to why. Furthermore, we found that three benign and two uncertain malignant GISTs by the AFIP criteria had mortality in this study. Therefore, further efforts to improve the diagnostic criteria to trace the malignant potential of GISTs are needed.
We found tumors with invasion or metastasis at the time of diagnosis in 13.7%. As expected, the tumors, which were confined to the original organs, revealed a better chance of survival than tumors with local invasion or metastasis. Any tumors showing metastatic lesion were classified into high risk or malignant GISTs in this study.
From the results of our study, we found several different epidemiologic characteristics of GISTs in Korea. Male gender was related with poor patient's survival in univariate analysis, but not in multivariate analysis. Male gender has been described as an independent adverse prognostic factor, (11) but not fully consistent. The average patient's age was 57.83 yr, and results in patients older than 60 yr indicated significant poor survival rates. The patient's age at the time of diagnosis has been described as a GIST prognosticator (11, 17, 21, 27). Extra-gastrointestinal tumors were found to be more common than in previous reports. We found 8.3% of extra-gastrointestinal malignant GISTs from the Korean NCI Registry and 10.1% of the data collected from 38 hospitals in this study. However, the proportion was reported to be 5% in previous reports (2). Extra-gastrointestinal GIST locations were omentum, mesentery, pelvis, intra-abdominal, retroperitoneum, abdominal wall and pancreas in our study. All the GISTs had been regarded as tumors with some malignant potential, but this is being challenged by recent data reflecting clinically insignificant and incidental GISTs. Microscopic GISTs were detected during autopsies of the stomach in 23% of patients older than 50 yr (28) and 35% of patients with gastric carcinoma (29). Incidental GISTs were found during operations of various diseases, but, except for the neurofibromatosis type 1 and Carney syndrome, no risk factors were identified. During the course of our study, we incidentally found GISTs in 8.2% and most commonly in the stomach during surgery for gastric carcinoma, but in patients with esophageal, colorectal, biliary tract, pancreatic, and hepatocellular carcinomas, gastrinoma, and ischemic colitis, GISTs were rarely found.
We found the prognostic significance in the pathologic findings of tumor necrosis and mucosal and vascular invasions, but they were rarely described in the pathology reports. From the results of this research, we recommend to describe these prognostic factors in daily practice, even though it is negative. We have three major limitations with regards to data of this study. The first one is incomplete information of chemotherapy. We reviewed the pathology reports with information of surgery. The treatment modality was not considered for survival analysis in this study. The second limitation is insufficient follow-up data. We analyzed the overall survival time but not the recurrence of diseases. The follow-up duration in our study was less than 7 yr. The third one is from the retrospective analysis of pathology reports. We could not randomize the treatment modality. Even though these limitations, we have concluded that the classification of GISTs based on original tumor location together with size and mitosis is more valuable to predict the prognosis. In addition, male gender, age and stage should be considered as factors for risk stratification of GISTs. Additionally, we recommend the description of the immunohistochemical results of CD34 and s-100 protein as well as histologic parameters such as tumor necrosis, mucosal and vascular invasions in pathology reports for daily practice.
Recently, many researchers focus on the significance of c-kit and PDGFRA mutation in predicting the response to tyrosine kinase inhibitor therapy (30) and the correlation of response to the therapy with type of c-kit mutation in GISTs (13, 25). Because the biologic behavior of GISTs is different in different locations and prognosis of low and intermediate risk tumors is uncertain, the genetic analysis of GISTs might be valuable, when assessing the prognosis of GISTs as well as the efficacy of therapy.
Figures and Tables
 | Fig. 1Univariate survival analyses of variables: gender (A, P=0.026), age (B, P=0.006), location (C, P=0.000), tumor size (D, P=0.000) and mitosis (E, P=0.000) are significantly correlated with patient's survival. |
 | Fig. 2Univariate analysis of variables of microscopic findings: Tumor necrosis (A), vessel invasion (B), mucosal invasion (C) and histologic type (D) are significantly correlated with patient's survival (P=0.000, 0.000, 0.000, 0.016, respectively). |
 | Fig. 3Univariate analysis of variables of immunohistochemical findings: CD34 expression (A) inversely relates with a patient's survival (P=0.001) in contrast to poor survival of GISTs with S-100 protein expression (B) (P=0.000). However α-smooth muscle actin expression (C) is not correlated with patient's survival (P=0.573). |
 | Fig. 4Univariate survival analysis of variables of diagnostic criterias: Both of the NIH criteria (A) and the AFIP criteria (B) are significantly correlated with patient's survival (P=0.000 & P=0.000). |
Table 1
Changes in incidence of malignant GISTs compared with all malignant tumors from 1999 to 2005: Data of Korean National Cancer Registry
References
1. Schaldenbrand JD, Appelman HD. Solitary solid stromal gastrointestinal tumors in von Recklinghausen's disease with minimal smooth muscle differentiation. Hum Pathol. 1984. 15:229–232.

2. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002. 33:459–465.

3. Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001. 438:1–12.
4. Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after sti-571. Hum Pathol. 2002. 33:466–477.

5. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. Pkctheta expression in gastrointestinal stromal tumor. Mod Pathol. 2006. 19:1480–1486.
6. Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M. A novel monoclonal antibody against dog1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008. 32:210–218.

7. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. Pdgfra activating mutations in gastrointestinal stromal tumors. Science. 2003. 299:708–710.

8. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of gists at different sites and their differential diagnosis with a reference to cd117 (kit). Mod Pathol. 2000. 13:1134–1142.

9. Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005. 103:821–829.
10. Tryggvason G, Gislason HG, Magnusson MK, Jonasson JG. Gastrointestinal stromal tumors in Iceland, 1990-2003: the Icelandic gist study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005. 117:289–293.

11. Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005. 100:162–168.

12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006. 23:70–83.

13. Hornick JL, Fletcher CD. The role of kit in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007. 38:679–687.

14. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. Gastrointestinal stromal tumors in Koreans: it's incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005. 20:977–984.

15. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006. 130:1466–1478.

16. Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RM, Hogendoorn PC. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer. 2005. 41:2868–2872.

17. Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006. 114:192–200.

18. Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002. 33:478–483.

19. Cho MY, Kang YK, Kim KM, Chang HK, Chang HJ, Chang MS, Kim JM, Kang DY, Park C, Sohn JH. Porposal for creating a guideline for cancer registration of the gastrointestinal tumors (i). Korean J Pathol. 2008. 42:140–150.
20. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008. 39:1411–1419.

21. Hou YY, Lu SH, Zhou Y, Xu JF, Ji Y, Hou J, Qi WD, Shi Y, Tan YS, Zhu XZ. Predictive values of clinical and pathological parameters for malignancy of gastrointestinal stromal tumors. Histol Histopathol. 2009. 24:737–747.
22. Huang HY, Li CF, Huang WW, Hu TH, Lin CN, Uen YH, Hsiung CY, Lu D. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007. 141:748–756.

23. Lasota J, Kopczynski J, Sarlomo-Rikala M, Schneider-Stock R, Stachura T, Kordek R, Michal M, Boltze C, Roessner A, Stachura J, Miettinen M. Kit 1530ins6 mutation defines a subset of predominantly malignant gastrointestinal stromal tumors of intestinal origin. Hum Pathol. 2003. 34:1306–1312.
24. Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, Robson M, Maki R, Brennan MF, Ladanyi M, DeMatteo RP, Besmer P. Association of kit exon 9 mutations with nongastric primary site and aggressive behavior: kit mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003. 9:3329–3337.
25. Park CK, Lee EJ, Kim M, Lim HY, Choi DI, Noh JH, Sohn TS, Kim S, Kim MJ, Lee HK, Kim KM. Prognostic stratification of high-risk gastrointestinal stromal tumors in the era of targeted therapy. Ann Surg. 2008. 247:1011–1018.
26. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005. 29:52–68.
27. Woodall CE 3rd, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC 2nd. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg. 2009. 144:670–678.

28. Agaimy A, Wunsch PH, Hofstaedter F, Blaszyk H, Rummele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (gist tumorlets) are common in adults and frequently show c-kit mutations. Am J Surg Pathol. 2007. 31:113–120.

29. Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006. 37:1527–1535.

30. Steigen SE, Eide TJ, Wasag B, Lasota J, Miettinen M. Mutations in gastrointestinal stromal tumors--a population-based study from Northern Norway. APMIS. 2007. 115:289–298.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download