Abstract
The purpose of our study was to determine the most accurate analytic method to define in vitro chemosensitivity, using clinical response as reference standard in prospective clinical trial, and to assess accuracy of adenosine triphosphate-based chemotherapy response assay (ATP-CRA). Forty-eight patients with chemo-naïve, histologically confirmed, locally advanced or metastatic gastric cancer were enrolled for the study and were treated with combination chemotherapy of paclitaxel 175 mg/m2 and cisplatin 75 mg/m2 for maximum of six cycles after obtaining specimen for ATP-CRA. We performed the receiver operator characteristic curve analysis using patient responses by WHO criteria and ATP-CRA results to define the method with the highest accuracy. Median progression free survival was 4.2 months (95% confidence interval [CI]: 3.4-5.0) and median overall survival was 11.8 months (95% CI: 9.7-13.8) for all enrolled patients. Chemosensitivity index method yielded highest accuracy of 77.8% by ROC curve analysis, and the specificity, sensitivity, positive and negative predictive values were 95.7%, 46.2%, 85.7%, and 75.9%. In vitro chemosensitive group showed higher response rate (85.7% vs. 24.1%) (P=0.005) compared to chemoresistant group. ATP-CRA could predict clinical response to paclitaxel and cisplatin chemotherapy with high accuracy in advanced gastric cancer patients. Our study supports the use of ATP-CRA in further validation studies.
Prediction of chemotherapy response before application to the patients may improve response to chemotherapy and reduce toxicity and the cost of care, providing tailored treatment to individual patients. Chemosensitivity assays refer to any in vitro laboratory analysis that are performed specifically to evaluate whether tumor growth is inhibited by a known chemotherapy drug (1). Ideal in vitro chemosensitivity testing should be reproducible, feasible with small amount of tissue, and the result should be available fast with high accuracy in predicting clinical response. Various chemosensitivity and resistance assays have been developed (2-4) but few have gained enough evidence to be utilized in clinical practice due to poor success rate, ambiguous criteria for defining in vitro sensitivity, prolonged turnaround time and lack of large, randomized trials comparing assay-guided therapy versus empirical therapy (5). American Society of Clinical Oncology Technology Assessment does not support use of any chemotherapy sensitivity and resistance assays for oncology practice (1).
Adenosine triphosphate (ATP)-based chemosensitivity test uses intracellular ATP level, to detect viability of cells under various concentrations of chemotherapeutic agents (6). It was reported to be more reproducible, feasible with smaller amount of tissue required and clinical application has been reported in melanoma, ovarian, and breast cancers (7-10), some studies showing correlation of in vitro response with clinical response and survival. However, few studies used predefined specific criteria for defining in vitro chemosensitivity, using clinical response in prospective clinical trials as a reference standard.
We used a modified method of ATP-based chemotherapy response assay (ATP-CRA) which adopted ultra-low attachment culture plates to inhibit the growth of normal cells to increase feasibility of the test using smaller amount of tissue and to shorten turnaround time (11, 12). This modified ATP-CRA has been tested and validated in various cancers including ovarian, breast and lung cancers (12-14), however, specific in vitro criteria defining chemosensitivity was not defined in prospective clinical trial and heterogenous methods were used in each study. The purpose of our study was to determine the most accurate analytic method to define in vitro chemosensitivity, using clinical response as reference standard in prospective clinical trial, and to assess accuracy of ATP-CRA using the method defined. We also set out to test the hypothesis that in vitro chemosensitivity could predict clinical outcomes in terms of response rate, progression free survival (PFS) and overall survival (OS) in advanced gastric cancer.
This trial was designed to define accuracy of ATP-CRA test in gastric cancer patients receiving paclitaxel and cisplatin chemotherapy, by comparing clinical response and ATP-CRA results in prospective, multi-center clinical trial. Primary end point was to assess accuracy of ATP-CRA results, and secondary endpoint was to find best method of defining in vitro chemosensitivity. Sample size was calculated to show sensitivity or specificity of ATP-CRA was more than 80%, to be 62 (α=0.05, δ=0.10). Considering 25% technical failure rate and 15% clinical drop out rate, total of 90 patients were to be enrolled.
Patients with chemotherapy-naïve, histologically or cytologically proven, metastatic or locally advanced gastric cancer not amenable to curative resection were enrolled for the study. Patient had to have at least 50 mg (endoscopic biopsy) or 250 mg (surgical biopsy) of tumor obtained, or at least 500 mL of ascites or pleural effusion, with more than 30% tumor cells to be included in the trial. Other inclusion criteria included age between 18-70, Karnofsky performance status: 60-100, bidimensionally measurable disease with more than 20 mm in computed tomography (CT) scan or 10 mm in radiography or physical examination and at least 12 weeks of life expectancy. Adjuvant chemotherapy given 6 months prior to enrollment was allowed and radiotherapy was allowed if the portal was outside of the measurable disease, with more than 4 weeks interval before enrollment. Patients who had active central nervous system metastasis, active infection, or other serious illness or medical conditions were excluded. Patients with less than 30% of tumor cells in malignant effusion were excluded from the study. This study was approved by local institutional review board (IRB) and all participants signed informed consent before enrollment. IRB approval numbers of participating centers are as follows; Seoul National University Bundang Hospital H-0306/104-009, Korea University Anam Hospital AN0328-001, Yeungnam University Medical Center 04-31-10, Seoul National University Boramae Hospital 06-2003-07, Seoul Paik Hospital 05-04-42, Korea Cancer Center Hospital 04-22(4), Seoul National University Hospital H-0306/104-009.
After obtaining informed consent for the study, tumor tissues were obtained by endoscopic biopsy, paracentesis of ascites, or excisional biopsy. Tumors were kept in transport media, containing Hank's balanced salt solution (HBSS, Gibco, Rockville, MD, USA), containing 100 IU/mL penicillin, 100 µg/mL streptomycin, 100 µg/mL gentamicin and 2.5 µg/mL amphotericin B and 5% fetal bovine serum, and transported to central laboratory within 24 hr of procedure. Tumor cells were separated by previously described method (11). Briefly, tissues were washed, quantified, minced and then white blood cells and red blood cells were removed using Ficoll gradient centrifugation at 400 g for 15 min and using magnetic bead containing anti-CD45 antibody (Miltenyi Biotech, Auburn, CA, USA). Tumor cells were diluted and seeded in triplicate to a 96-well ultra-low attachment plate, at 2,000-20,000 cells/well. 100 µL of paclitaxel and cisplatin were added to the seeded cell cultures at ×5, 1, 0.5, 0.2, 0.1 times the individual test drug concentrations (2.5 µg/mL for cisplatin, 3 µg/mL for paclitaxel) determined by previous method (15, 16), and incubated for 48 hr in a CO2 incubator. ATP in the cell lysates of treated and untreated control was measured using flash type luminescence measurements (Roche, Mannheim, Germany), and the inhibition rate was determined as the rate of ATP luminescence reduction in the treated group compared with the untreated control.
After obtaining tumor tissue, patients received combination chemotherapy consisting of paclitaxel 175 mg/m2 and cisplatin 75 mg/m2, on day 1 every 3 weeks for a maximum of 6 cycles. Tumor response was assessed by WHO criteria (17), using CT scan taken every 2 cycles. Both laboratory technicians and physicians were blinded to ATP-CRA results or clinical results. The chemosensitivity was assessed using 1) chemosensitivity index (CI) method, CI calculated by adding the percentage of tumor growth inhibition at each drug concentration tested (CI=[100×# concentration tested]-SUM [% cell suppression at given concentrations among 0.1×-5×]) (6); 2) comparison of in vitro area under the curve (AUC) at IC50, drug concentration that achieve 50% growth inhibition in vitro, vs. clinical AUC (18, 19); 3) single concentration arbitrary criteria, which uses cut-off value of in vitro inhibition rate determined by Fisher's exact test, which discriminates clinical responders and non-responders (20); and 4) using mean value of growth inhibition rate using patient database (21). Patients were considered to be chemo-sensitive if they were sensitive to either one of the two drugs, defined by cut off values determined as above.
Receiver operating characteristic (ROC) curve analysis and Fisher's exact test were performed to select analytic method with highest accuracy. By the method chosen, 2×2 table was constructed yielding sensitivity, specificity, positive and negative predictive value, and accuracy was calculated as proportion of true positive and true negative patients out of whole patients.
Survival curves were constructed using the Kaplan-Meier method. The log-rank test was used to compare survival probabilities between in vitro responders and non-responders. All statistical calculations were carried out by independent statisticians at CMIC Korea, Ltd., using SAS 8.1 (SAS Institute, Cary, NC, USA) and the SPSS Windows program version 15 (SPSS, Chicago, IL, USA). P values <0.05 were considered statistically significant.
From September 2003 to January 2006, a total of 71 patients signed informed consent and underwent biopsy from seven centers. Of the 71 patients, 23 were deemed ineligible due to not enough tumor cells (n=10), bacterial contamination (n=3), violation of enrollment criteria (n=6), and not receiving planned treatment (n=4), rendering 48 patients enrolled. Majority of specimens were endoscopic biopsies of primary tumor (46 out of 48) and remaining two were obtained from excisional biopsy and ascites. The study was originally designed to enroll 62 evaluable patients, but the trial was terminated and analyzed after enrolling 48 patients due to poor patient accrual.
The median age of the patients were 57 yr (range, 31-70) and other clinical characteristics are summarized in Table 1. No patients had received adjuvant chemotherapy or radiotherapy before enrollment. Of the 48 patients, 11 were inevaluable (4 due to inaccurate dose of chemotherapy, 6 were lost to follow up before response assessment, 1 culture failure and 1 secondary bacterial contamination during the assay) and 36 patients were evaluable for both in vitro and in vivo responses.
Thirty-six evaluable patients received total of 152 cycles of chemotherapy, median number of cycles received was four (1-6). Among 36 evaluable patients, response rate was 36.1% with 13 patients achieving partial response, 12 stable disease and 11 patients with progressive disease. Twenty-seven patients received second line chemotherapy upon progression. Median PFS was 4.2 months (95% CI: 3.4-5.0) and median OS was 11.8 months (95% CI: 9.7-13.8) for all enrolled patients.
Of the 48 patients enrolled, 46 specimens yielded chemosensitivity results, with two test failures; one secondary bacterial contamination during the assay, one culture failure. The success rate on the intention-to-assay basis was 75.4% (46 of 61, excluding ten patients with violation of enrollment criteria and who did not receive planned treatment). If the samples met all the inclusion criteria of more than 50 mg (endoscopic biopsy) or 250 mg (surgical biopsy), or at least 500 mL of ascites or pleural effusion, with more than 30% tumor cells, the success rate was as high as 95.8% (46 of 48 enrolled patients). Chemosensitivity was determined using four different methods described in the methods and ROC curve analysis was performed to define the method with highest accuracy (Table 2). Chemosensitivity index method, which used ×1.0 and ×5.0 concentrations and cut-off value of 49.9, were chosen as the method with highest accuracy of 77.8% (Table 2, Fig. 1). This method would define a patient as in vitro sensitive, if the sum of percentage of cell death at ×1.0 and ×5.0 times peak plasma concentrations of either paclitaxel or cisplatin were below cut-off value of 49.9.
Using chemosensitivity index method defined above, specificity of ATP-CRA was 95.7% (95% CI: 77.2-99.9%); sensitivity 46.2% (95% CI: 19.2-74.9%); positive predictive value 85.7% (95% CI: 42.1-99.6%); and negative predictive value was 75.9% (95% CI: 55.1-89.3%), respectively (Table 3). Other methods such as chemosensitivity index method using ×1 and ×5 concentrations, cut off point of 52.3, single concentration and arbitrary criteria at cisplatin ×1 or paclitaxel ×1 concentrations, cut off point of 52.7 yielded accuracy above 70% (Table 2).
Using the selected chemosensitivity index method and cutoff point, seven patients were defined as in vitro sensitive (S) and 29 patients as resistant (R). S group and R group showed similar baseline characteristics such as age, sex, stage, performance, and whether they received second line chemotherapy (Table 4). S group showed statistically significantly higher response rate compared to R group (85.7% vs. 24.1%, P=0.005). Progression free survival (median: 4.3 vs. 4.5 months, mean: 5.5 vs. 4.4 months, P=0.60) and overall survival (8.4 vs. 12.3 months, P=0.08) difference did not reach statistical significance (Fig. 2).
We found that chemosensitivity index method using ×1 and ×5 times peak plasma concentration and cut-off value of 49.9, was the best method of defining in vitro chemosensitivity with accuracy of 77.8%, specificity of 95.7% and sensitivity of 46.2%. Our study also showed that ATP-CRA was feasible with small amount of tissue, mainly endoscopic biopsies, in multicenter setting with success rate of 75.4% (intention to assay) or 95.8% (enrolled patients), with turnaround time of less than 7 days.
Our results compare favorably with others. O'Meara et al. reported the results of ATP chemosensitivity assay in ovarian cancer, with sensitivity and specificity of 68.8%, 74.3%, accuracy of 70.7% and positive and negative predictive value of 83% and 56.5%, respectively (22). They also performed ROC curve analysis to define the most accurate method and reported criteria to be cell kill of ≥45% at the dose of 0.5 times peak plasma concentration for paclitaxel. Modified ATP-CRA used in our study has been tested in various cancers including ovarian, breast, and lung cancers (12-14), and these studies also reported similar or higher accuracy, specificity, sensitivity and positive and negative predictive values. However, most of the studies have used mixed population of patients and included heterogeneous treatment, such as first line and second line chemotherapy or neoadjuvant and metastatic setting, or were not designed prospectively. Most of the studies with ATP chemosensitivity assays were performed in ovarian cancer where maximum debulking surgery is routinely performed prior to chemotherapy and cut-off criteria for in vitro chemosensitivity was determined using progression free or overall survival, or CA-125 response, instead of clinical response which requires measurable disease (8, 13, 22).
In vitro sensitive patients (S-group) defined by ATP-CRA test showed increased responses rate compared to in vitro resistant patients (R-group), more than three times that of R-group (85.7% vs. 24.1%). Although the response rate was higher in the S-group, there were no statistically significant differences in progression free or overall survival in our patients. S-group showed tendency for shorter overall survival compared to R-group. Our results are in contrast to others reporting correlation of in vitro chemosensitivity with progression free and/or overall survival, where in vitro chemosensitive patients showed longer survivals (8, 10, 12). Possible explanations of this discrepancy could mainly be due to small number of patients enrolled, especially small numbers of in vitro chemosensitive (S) group. Our study could have been underpowered according to the initial statistical assumptions and this could lead to failure to distinguish differences in PFS and OS. Also, since we only used responders in defining in vitro sensitivity, value of stable disease could not have been taken into account and patients with non-responding but with stable disease would have been classified as R-group. Moreover, in vitro chemosensitivity could be a marker of aggressiveness, which may compensate for initial response achieved by chemotherapy. Our findings warrant further study including patients with stable disease as responders in defining in vitro chemosensitivity.
There are several limitations to our study. First, the study took almost three years to enroll 36 evaluable patients. Many samples were not enrolled due to bacterial contamination, inadequate amount of tissue especially during the first few months. Once the adequate tumor cells were isolated, success rate of ATP-CRA was 95.8%. Second, the study was originally designed to enroll 62 evaluable patients, to show sensitivity or specificity of ATP-CRA was more than 80% with type 1 error (α) of 0.05. We had to terminate our study early due to very poor accrual of patients, and it resulted in inadequate power to test the accuracy as planned initially. The type one error would be 0.067 with 36 evaluable patients, instead of 0.05 with planned 62 evaluable patients. Third, because the test accuracy was calculated using the criteria defined by ROC curve analysis, our study result needs validation in an independent cohort. We are currently enrolling patients with lung cancer, using the same paclitaxel plus cisplatin regimen, and expect to validate this method in this independent cohort. Fourth, the clinical response was evaluated in the participating centers by the investigators who were blind to the in vitro chemosensitivity results but there was no independent review of response evaluation, which may have been subject to biases.
Despite limitations, our study is one of the few attempts to define criteria for in vitro chemosensitivity using clinical response in a prospectively designed trial as reference standard. We enrolled homogenous population of chemo-naïve patients and used homogenous treatment to minimize other variables that could affect chemosensitivity.
Positive and negative predictive value of a diagnostic test depends strongly on the frequency of event, in this case, response rate. Even diagnostic test with 95% sensitivity and specificity would yield very low positive and negative predictive values if applied to a low prevalence event, whereas test with high specificity test would yield high negative predictive value in low prevalence event (23). Considering this statistical assumption, specificity of more than 95% but rather low sensitivity of 46.2% of our study support the use of ATP-CRA in selecting chemotherapeutic agent which would not benefit patients, in cancers with low response rate where cytotoxic chemotherapies benefit only selected patients. Most useful application of ATP-CRA would be for selecting adjuvant regimen, when chemosensitivity only can be assessed many years later in terms of disease free survival and selection of optimal chemotherapeutic agents could increase cure rate.
In conclusion, ATP-CRA can predict clinical response to paclitaxel and cisplatin chemotherapy with high success rate and accuracy in advanced gastric cancer patients. The higher response rate shown in chemosensitive group supports the use of ATP-CRA in further validation studies and assay-guided clinical trials.
Figures and Tables
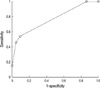 | Fig. 1Receiver operating characteristic curve of ATP-chemotherapy response assay using ×1 and ×5 times test drug concentrations of paclitaxel and cisplatin. |
 | Fig. 2Progression free and overall survival of in vitro sensitive versus in vitro resistant patients. (A) Progression free survival. (B) Overall survival. |
Table 2
Accuracy, sensitivity, specificity, positive predictive value, and negative predictive value according to the four methods of in vitro chemosensitivity assessment

References
1. Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR. American society of clinical oncology technology assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004. 22:3631–3638.

2. Von Hoff DD, Clark GM, Stogdill BJ, Sarosdy MF, O'Brien MT, Casper JT, Mattox DE, Page CP, Cruz AB, Sandbach JF. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983. 43:1926–1931.
3. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987. 47:936–942.
4. Gazdar AF, Steinberg SM, Russell EK, Linnoila RI, Oie HK, Ghosh BC, Cotelingam JD, Johnson BE, Minna JD, Ihde DC. Correlation of in vitro drug-sensitivity testing results with response to chemotherapy and survival in extensive-stage small cell lung cancer: a prospective clinical trial. J Natl Cancer Inst. 1990. 82:117–124.

5. Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004. 22:3618–3630.

6. Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, Gleiberman I, Caruso PA, Ricks SH, Untch M. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995. 55:5276–5282.
7. Cree IA, Neale MH, Myatt NE, de Takats PG, Hall P, Grant J, Kurbacher CM, Reinhold U, Neuber K, MacKie RM, Chana J, Weaver PC, Khoury GG, Sartori C, Andreotti PE. Heterogeneity of chemosensitivity of metastatic cutaneous melanoma. Anticancer Drugs. 1999. 10:437–444.

8. Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, Cree IA, Hepp H, Untch M. Correlation of drug response with the ATP tumorchemosensitivity assay in primary figo stage III ovarian cancer. Gynecol Oncol. 2000. 77:258–263.

9. Kurbacher CM, Cree IA, Brenne U, Bruckner HW, Kurbacher JA, Mallmann P, Andreotti PE, Krebs D. Heterogeneity of in vitro chemosensitivity in perioperative breast cancer cells to mitoxantrone versus doxorubicin evaluated by a microplate ATP bioluminescence assay. Breast Cancer Res Treat. 1996. 41:161–170.
10. Ugurel S, Schadendorf D, Pfohler C, Neuber K, Thoelke A, Ulrich J, Hauschild A, Spieth K, Kaatz M, Rittgen W, Delorme S, Tilgen W, Reinhold U. In vitro drug sensitivity predicts response and survival after individualized sensitivity-directed chemotherapy in metastatic melanoma: a multicenter phase ii trial of the dermatologic cooperative oncology group. Clin Cancer Res. 2006. 12:5454–5463.

11. Kang SM, Park MS, Chang J, Kim SK, Kim H, Shin D, Chung KY, Kim DJ, Sohn JH, Choi SH, Kim J, Yoon EJ, Kim JH. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res Treat. 2005. 37:223–227.

12. Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, Park MS, Chung KY, Lee HJ, Kim JH. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007. 109:1829–1835.

13. Han SS, Choi SH, Lee YK, Kim JW, Park NH, Song YS, Lee HP, Kang SB. Predictive value of individualized tumor response testing by atp-based chemotherapy response assay in ovarian cancer. Cancer Invest. 2008. 26:426–430.

14. Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, Choi HY, Kim HK, Park HK, Choi SH, Yoon EJ, Oh SY. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008. 17:19–26.

15. Bepler G, O'Briant K. In vitro chemosensitivity testing of human non-small cell lung cancer cell lines. Anticancer Res. 1998. 18:3181–3185.
16. Boddy AV, Griffin MJ, Sludden J, Thomas HD, Fishwick K, Wright JG, Plumner ER, Highley M, Calvert AH. Pharmacological study of paclitaxel duration of infusion combined with GFR-based carboplatin in the treatment of ovarian cancer. Cancer Chemother Pharmacol. 2001. 48:15–21.
17. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.

18. Cree IA, Kurbacher CM, Untch M, Sutherland LA, Hunter EM, Subedi AM, James EA, Dewar JA, Preece PE, Andreotti PE, Bruckner HW. Correlation of the clinical response to chemotherapy in breast cancer with ex vivo chemosensitivity. Anticancer Drugs. 1996. 7:630–635.

19. Kurbacher CM, Cree IA, Bruckner HW, Brenne U, Kurbacher JA, Muller K, Ackermann T, Gilster TJ, Wilhelm LM, Engel H, Mallmann PK, Andreotti PE. Use of an ex vivo ATP luminescence assay to direct chemotherapy for recurrent ovarian cancer. Anticancer Drugs. 1998. 9:51–57.

20. Sevin BU, Peng PZ, Perras JP, Ganjei P, Penalver M, Averette HE. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988. 31:191–204.

21. Bosanquet AG, Bell PB. Enhanced ex vivo drug sensitivity testing of chronic lymphocytic leukaemia using refined disc assay methodology. Leukemia research. 1996. 20:143–153.

22. O'Meara AT, Sevin BU. Predictive value of the ATP chemosensitivity assay in epithelial ovarian cancer. Gynecol Oncol. 2001. 83:334–342.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download