Abstract
Despite the prophylaxis and preemptive strategies using potent antiviral agents, cytomegalovirus (CMV) remains a major infectious cause of morbidity and mortality in allogeneic stem cell transplantation (SCT) recipients. Delayed immune reconstitution after SCT, such as cord blood and T-cell depleted SCT with the use of alemtuzumab, has been associated with an increased frequency of CMV disease as well as CMV reactivation. CMV disease involving central nervous system is an unusual presentation in the setting of SCT. We report a case of CMV ventriculoencephalitis after unrelated double cord blood SCT with an alemtuzumab-containing preparative regimen for Philadelphia-positive acute lymphoblastic leukemia.
Cytomegalovirus (CMV) infection still remains a major cause of morbidity and mortality in allogeneic stem cell transplantation (SCT) recipients. While CMV infection of the central nervous system (CNS) in acquired immune deficiency syndrome patients has been reported relatively frequently, it is an unusual presentation in allogeneic SCT recipients, but fatal in all cases (1-4). Recently, unrelated cord blood or T-cell depleted grafts have been increasingly used as an alternative source of hematopoietic stem cells. However, the use of these grafts has been associated with an increased frequency of unusual CMV infections.
Here, we report a case of CMV ventriculoencephalitis after unrelated double cord blood SCT with an alemtuzumab-containing preparative regimen for Philadelphia-positive acute lymphoblastic leukemia. The patient had recurrent CMV DNAemia despite long-term treatment with antiviral agents (foscarnet combined with ganciclovir) and anti-CMV immunoglobulin.
A 20-yr-old man underwent unrelated cord blood SCT using two cord blood units to treat Philadelphia-positive acute lymphoblastic leukemia. Each cord blood unit had 5/6 HLA compatibility. The patient was treated with total body irradiation (1,200 cGy), fludarabine (150 mg/m2), cytarabine (9 g/m2), and alemtuzumab (20 mg) as a preparative regimen. Graft-versus-host disease prophylaxis was attempted by administering tacrolimus plus mizoribine. The patient was CMV-seropositive at the time of transplantation. CMV serology test was not performed in the cord blood. For prophylaxis of CMV reactivation, acyclovir (10 mg/kg intravenously every 8 hr) and anti-CMV immunoglobulin (150 mg/kg intravenously biweekly) were given from day -7 until engraftment. The patients achieved successful neutrophil and platelet engraftment on day 27 and 51, respectively.
On day 33, CMV DNAemia was detected by real-time quantitative polymerase chain reaction (RQ-PCR) as 3,775 copies/mL and the viral load increased steadily thereafter. The patient was treated preemptively for CMV DNAemia with foscarnet (60 mg/kg intravenously every 12 hr for 7 days, followed by 90 mg/kg intravenously daily) from day 39 to day 93. During this period, CMV DNAemia decreased to low levels (<500 copies/mL). Foscarnet had been chosen, instead of ganciclovir, since it had less potential bone marrow toxicity. Thirteen days after discontinuation of the foscarnet, CMV retinitis developed with an increase in CMV DNA titer. The foscarnet (90 mg/kg intravenously every 12 hr) and anti-CMV immunoglobulin (150 mg/kg intravenously biweekly) were re-administered. However, 7 days later, the CMV RQ-PCR titers increased despite the treatment. Then we added ganciclovir (5 mg/kg intravenously daily) on top of foscarnet (90 mg/kg intravenously daily). Clinically, the CMV retinitis improved and the CMV DNAemia resolved 40 days after the combination therapy.
On day 171, he was re-admitted complaining of progressive weakness of all four extremities. An increased titer of CMV RQ-PCR was noted again. The peripheral blood showed pancytopenia without leukemic cells; a WBC count of 1,160/µL, hemoglobin of 6.9 g/dL, and a platelet count of 6,000/µL. Despite administration of foscarnet and anti-CMV immunoglobulin, there was no improvement of CMV DNA-emia. Rather, peripheral neuropathy was in progress and mental status slowly deteriorated. He became inattentive and somnolent on day 185. A gadolinium-enhanced magnetic resonance imaging of the brain (Fig. 1) showed multiple nodularity with high signal intensities in the bilateral fronto-parietal subcortical white matter, periventricular white matter, and the basal ganglia in the T2-weighted and FLAIR images. Diffuse high signal changes in the T2-weighted and FLAIR images were also noted along the wall of the lateral ventricles, which were enhanced with gadolinium on the T1-weighted images. Lumbar puncture was performed on day 186; the results showed a protein concentrate of 66.5 mg/dL, a glucose concentrate of 62 mg/dL (serum glucose concentrate, 145 mg/dL), no white blood cells, and no red blood cells. The microbiology of the cerebrospinal fluid (CSF) was negative for bacteria, Mycobacterium species, and fungi. There was no skin lesion that suggested a varicella zoster virus infection, and the PCR analysis of the CSF for herpes simplex virus (type 1 & 2) and human herpes virus 6 were all negative. However, the PCR analysis of the CSF was positive for CMV (Fig. 2). Based on the typical radiologic findings, the positive PCR for CMV from the CSF, and recurrent CMV DNAemia in the peripheral blood in spite of long-term antiviral therapy, a CMV ventriculoencephalitis had been diagnosed in the case. Eventually the patient died of progressive deterioration of CNS function on day 190. The clinical course and laboratory data of the patient are summarized in Fig. 3.
Recently, posttransplant immune reconstitution after SCT varies because of availability of alternative donor and variety of preparative regimens and graft manipulations. The unrelated cord blood SCT and haploidentical transplantation themselves may be correlated with a high incidence of CMV reactivation, when compared to matched sibling or unrelated SCT (5, 6), as well as the use of antithymocyte globulin, fludarabine, and alemtuzumab (humanized monoclonal CD52 antibody) as part of the preparative regimen (7-13). In particular, the combination of fludarabine and alemtuzumab might cause a higher risk of CMV infection and earlier CMV infection following allogeneic SCT compared to other preparations (8). Chakrabarti et al. (7) suggested that the patients receiving alemtuzumab, a total dose of 100 mg, had a higher incidence of CMV infection; a lower dose of alemtuzumab might be related with a reduced risk of CMV infection. Our patient received a much lower dose of alemtuzumab, a total dose of 20 mg. However, he had a recurrent CMV reactivation and finally developed CMV retinitis and ventriculoencephalitis.
A combination of intravenous foscarnet and ganciclovir has been advocated for treatment of single drug-resistant CMV disease (14, 15). We tried the combination regimen of foscarnet and ganciclovir, expected to have synergistic effects, for the treatment of CMV retinitis because of two reasons; 1) we used the combination of fludarabine and alemtuzumab for CBT preparation, which might have made the patient highly susceptible to CMV reactivation; 2) CMV retinitis developed after treatment with CMV DNAemia despite the recent prolonged treatment with foscarnet.
Clinically, the treatment failure of CMV disease with antiviral drugs may be associated with antiviral resistance and/or inadequate penetration of the drug into the infected tissue (1, 3, 16, 17). The recurrent CMV DNAemia and progressive CMV disease unresponsive to antiviral agents and anti-CMV immunoglobulin for about 150 days, suggest antiviral resistant-mutant CMV in the case. However, it is the limitation of the current study that we could not perform drug susceptibility analysis, CMV genotyping or phenotyping for drug resistance-associated mutations, or measurement of the drug levels in the plasma and CSF.
In summary, more aggressive prophylaxis as well as preemptive therapy for CMV infection and more diligent monitoring for progression to CMV diseases should be considered for a certain proportion of SCT recipients with multiple risk factors of delayed immune reconstitution.
Figures and Tables
Fig. 1
Brain magnetic resonance images. The T1-weighted image (A) showed periventricular enhancement of subependymal regions (▼), which indicated the ventriculoencephalitis. The FLAIR image (B) showed hyperintense lesions (*) in the hippocampus bilaterally, which can be seen in diffuse encephalitis.
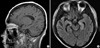
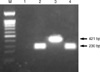
Fig. 2
Gel electrophoresis of DNA fragments amplified by multiplex PCR. DNA was extracted using a QIAamp DNA Mini Kit (QIAGEN, Germany). Then, the PCR for CMV and HHV-6, described previously by Park et al. (18), was performed as positive control. Template DNAs for the different viruses were isolated from each CMV AD169 (ATCC VR-538) and HHV6-B (HST strain, provided by K Tanaka-Taya, Osaka University Graduate School of Medicine, Osaka, Japan). Lanes: M, 100 bp DNA ladder; 1, negative control; 2, CMV; 3, HHV-6; 4, CSF of the patient.
References
1. Julin JE, van Burik JH, Krivit W, Webb C, Holman CJ, Clark HB, Balfour HH Jr. Ganciclovir-resistant cytomegalovirus encephalitis in a bone marrow transplant recipient. Transpl Infect Dis. 2002. 4:201–206.

2. Zeiser R, Grüllich C, Bertz H, Pantazis G, Hufert FT, Bley TA, Finke J. Late cytomegalovirus polyradiculopathy following haploidentical CD34+-selected hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004. 33:243–245.

3. Miller GG, Boivin G, Dummer JS, McConnell T, Becher MW, Kassim A, Tang YW. Cytomegalovirus ventriculoencephalitis in a peripheral blood stem cell transplant recipient. Clin Infect Dis. 2006. 42:e26–e29.

4. Seo SK, Regan A, Cihlar T, Lin DC, Boulad F, George D, Prasad VK, Kiehn TE, Polsky B. Cytomegalovirus ventriculoencephalitis in a bone marrow transplant recipient receiving antiviral maintenance: clinical and molecular evidence of drug resistance. Clin Infect Dis. 2001. 33:e105–e108.

5. Takami A, Mochizuki K, Asakura H, Yamazaki H, Okumura H, Nakao S. High incidence of cytomegalovirus reactivation in adult recipients of an unrelated cord blood transplant. Haematologica. 2005. 90:1290–1292.
6. Tomonari A, Iseki T, Ooi J, Takahashi S, Shindo M, Ishii K, Nagamura F, Uchimaru K, Tani K, Tojo A, Asano S. Cytomegalovirus infection following unrelated cord blood transplantation for adult patients: a single institute experience in Japan. Br J Haematol. 2003. 121:304–311.

7. Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, Chakraverty R, Marshall T, Osman H, Mahendra P, Craddock C, Waldmann H, Hale G, Fegan CD, Yong K, Goldstone AH, Linch DC, Milligan DW. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002. 99:4357–4363.

8. Bainton RD, Byrne JL, Davy BJ, Russell NH. CMV infection following nonmyeloablative allogeneic stem cell transplantation using Campath. Blood. 2002. 100:3843–3844.

9. Mohty M, Faucher C, Vey N, Stoppa AM, Viret F, Chabbert I, Chabannon C, Bouabdallah R, Ladaique P, Collet L, Zandotti C, Maraninchi D, Blaise D. High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant. 2000. 26:251–255.

10. Redy V, Pollock BH, Sharda S, Hopkins J, Weeks F, Manion K, Roque D, Leather HL, Finiewicz KJ, Khan SA, Mehta P, Moreb JS, Wingard JR. GVHD and CMV antigenemia after allogeneic peripheral blood stem cell transplantation: comparison between myeloablative and nonmyeloablative (mini) conditioning regimens. Blood. 2000. 96:191a.
11. Junghanss C, Boeckh M, Carter R, Sandmaier BM, Maloney D, Chauncey T, McSweeney PA, Corey L, Storb R. Incidence of herpesvirus infections following nonmyeloablative allogeneic stem cell transplantation. Blood. 2000. 96:188a.
12. Schilling PJ, Vadhan-Raj S. Concurrent cytomegalovirus and pneumocystis pneumonia after fludarabine therapy for chronic lymphocytic leukemia. N Engl J Med. 1990. 323:833–834.

13. Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006. 43:16–24.

14. Mylonakis E, Kallas WM, Fishman JA. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin Infect Dis. 2002. 34:1337–1341.

15. Anduze-Faris BM, Fillet AM, Gozlan J, Lancar R, Boukli N, Gasnault J, Caumes E, Livartowsky J, Matheron S, Leport C, Salmon D, Costagliola D, Katlama C. Induction and maintenance therapy of cytomegalovirus central nervous system infection in HIV-infected patients. AIDS. 2000. 14:517–524.

16. Fletcher C, Sawchuk R, Chinnock B, de Miranda P, Balfour HH Jr. Human pharmacokinetics of the antiviral drug DHPG. Clin Pharmacol Ther. 1986. 40:281–286.

17. Wagstaff AJ, Bryson HM. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994. 48:199–226.
18. Park ST, Kim SH, Lee DG, Choi JH, Shin WS, Kim TG, Paik SY, Kim CC. Detection of lymphotropic herpesviruses by multiplex polymerase chain reaction. Korean J Microbiol. 2001. 39:226–228.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download