Abstract
To clarify the characteristics of the virulence factors (VFs) of ciprofloxacin resistant Escherichia coli (CFRE) with acute uncomplicated cystitis (AUC), we determined the VFs and the phylogenetic background of all 54 CFRE strains and the 55 randomly selected ciprofloxacin sensitive E. coli strains (CFSE) from patients with AUC in 22 Korean hospitals. The prevalence of the VFs was as follows: fimA, papEF, papGIII, sfaI, dafaBC, cnf1, and hlyA were presented in 96%, 54%, 68%, 91%, 49%, 72%, and 29% of the samples, respectively. The expressions of papEF, cnf1, and hlyA were significantly more prevalent in the CFSE. Moreover, the expressions of cnf, and papEF significantly reduced the risk of ciprofloxacin resistance. The CFSE was also marginally associated with the group B2 (P=0.05). Although the presence of pyuria and a previous cystitis history were not related with the phylotyping and the expressions of VFs, group B2, and fimA and papEF were more expressed in the younger age patients (P<0.05). In conclusion, the CFRE exhibits a selective loss of VFs and the non-B2 phylotype in Korean AUC patients. The group B2 and the presence of fimA and papEF are associated with a younger age of AUC patients.
Acute uncomplicated cystitis (AUC) is the most commonly encountered bacterial infections in healthy women and fluoroquinolone treatment has been a standard therapy for it (1). However, a trend toward increasing drug resistance among Escherichia coli (E. coli) has complicated the treatment of AUC over the past few years (2).
Most of these infections are caused by distinctive E. coli strains, and these strains possess virulence factors (VFs) to overcome host defenses and injure or invade host tissues so that the E. coli persist and multiply within the host's urinary tract (3). Several investigations have shown that the ciprofloxacin resistant E. coli strains (CFRE) display overall reduced virulence, whereas the ciprofloxacin susceptible E. coli strains (CFSE) are more virulent (4-7). Some epidemiological studies have also reported that CFRE isolates exhibit a different phylotype than do their susceptible counterparts. However, the previous studies have major limitations for their findings to be generally accepted. First of all, the small numbers of the CFRE isolates may limit the ability to characterize the resistant strains because of the increased likelihood of type I and II errors (7). Second, because the expression patterns of VF and the phylotyping are different among different countries, the isolates from western countries have some limitations to explain the phylotyping and VFs in Asia. For example, Russian urinary tract infection isolates belonged more often to phylogenetic group A and they possessed fewer virulence genes than did the Norwegian isolates (9, 10). Third, the available studies were a mix of data from uncomplicated and complicated cystitis. However, the characteristics of AUC are different from the complicated cystitis according to the antibiotic resistance, the drug treatment responses and the associated diseases. We must separately characterize the two patterns of cystitis in a study that focuses on their phylotyping and their virulence expressions. Fourth, the absence of the clinical or epidemiological data of the host in the previous studies does not allow assessing the impact of the host on certain VF profiles or on the phylotyping from the infection.
To clarify the clinical characteristics of the ciprofloxacin resistance in patients with AUC, we analyzed the VFs, the resistance phenotype and the clinical characteristics for 54 samples of ciprofloxacin resistant strains from patients with AUC and 55 randomly selected ciprofloxacin sensitive strains from patients with AUC in 22 Korean hospitals.
All the female patients between 18 and 65 yr old and who presented with symptoms of dysuria, urgency, frequency or a combination of these between May and October 2006 were included in this study. The exclusion criteria were symptoms of or predisposing factors for complicated cystitis. These women presented to outpatient urological clinics and they were not hospitalized. The women who had received antimicrobial agents in the 4 weeks previous to the study were excluded. A clean-catch midstream urine sample was examined for the presence of leukocytes by microscopy, and then it was sent to a central laboratory for culture. Only one specimen per patient was accepted. No mixed infections were accepted. The E. coli included in the study were from cultures yielding ≥103 colony forming unit (CFU)/mL (2).
In total, 225 E. coli isolates were prospectively collected from the urine samples of the female outpatients with uncomplicated cystitis in 22 hospitals of Korea. The minimum inhibitory concentration (MIC) of ciprofloxacin resistance was determined by the agar dilution method on Mueller-Hinton agar (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) as recommended by the NCCLS (2). All 54 samples with ciprofloxacin resistance and the 55 randomly selected ciprofloxacin sensitive samples were included. For the total 109 isolated samples, 6 of the patients' medical records were not available for clinical data analysis. The numbers of white blood cells (WBC) in the urine were dichotomized into 1-4 WBC and ≤5 WBC per high power field. The numbers of cases of recurrent cystitis were also dichotomized into no recurrence and recurrence within a year after the first bout of AUC.
One E. coli colony was harvested from the characterized plate and the bacteria were grown at 37℃ in Luria-Bertani broth. The bacterial DNA was extracted by using the Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA). The E. coli isolates were grouped into phylogenetic biotypes (A, B1, B2, and D) according to the presence of the chuA and yjaA genes and TspE4.C2 by performing multiplex polymerase chain reaction (PCR) (Table 1) (11). PCR was performed with 25 µL of the following reaction mixture: 12.5 µL of 2 times GoTaq Green Master mixture (Promega), 20 pM of each primer and 1 µL of the DNA template. The amplification conditions were as follows: each step consisted of 95℃ for 30 sec, 55℃ for 30 sec and 72℃ for 30 sec, and there was a final extension step of 7 min at 72℃. Thirty cycles were performed for amplification.
We also examined for 7 VFs (fimA, papGIII, papEF, cnf1, hlyA, sfaI, and dafaBC) by performing a single polymerase chain reaction (Table 1). The amplification conditions were as follows: each step consisted of denaturation at 95℃ for 30 sec, annealing at 53℃ for fimA and papGIII, 55℃ for cnf1, 57℃ for hlyA, papEF, and sfaI, and 59℃ for 30 sec for dafaBC and extension at 72℃ for 30 sec. Twenty five-thirty cycles were performed for amplification.
Statistical analysis was performed by using Fisher's exact test, chi-square tests, Student's t-tests and the Mann-Whitney test. Multiple variables were assessed as predictors of the categorical outcomes by multivariable logistic regression analysis. P values ≤0.05 were considered statistically significant.
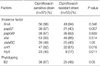
The prevalence of VFs were as follows; fimA, papEF, papGIII, sfaI, dafaBC, cnf1, and hlyA was present in 96%, 54%, 68%, 91%, 49%, 72%, and 29% of the samples, respectively (Fig. 1). The expressions of papEF, cnf1, and hlyA were significantly more prevalent in the CFSE than in the CFRE (P=0.007, P=0.018, P=0.011, respectively), whereas the expression of fimA, dafaBC, papGIII, and sfaI were not (P=0.346, P=1.0, P=0.839, P=0.514, respectively) (Table 2). The phylotype B2, D, A, and B1 was present in 57%, 26%, 16%, and 1% of the samples, respectively (Fig. 1). The CFSE was marginally associated with the group B2 (P=0.05) (Table 2). Presence of pyuria and a previous history of AUC within the recent year were not related with the phylotyping and the all virulence factors (Table 3). The expressions of phylotype groups B2, papEF, and fimA were associated with a lower absolute patient age (P=0.03, P=0.002, P=0.03, respectively) (Table 4). On the logistic regression model, the expressions of cnf1 and papEF reduced the ciprofloxacin resistance risk (odds ratio [OR] 0.237, 95% confidence interval [CI] 0.07-0.79 for cnf1; OR 0.40, 95% CI 0.16-0.96 for papEF) (Table 5). However, the phylogenetic background did not increase the risk of ciprofloxacin resistance (OR 1.843, 95% CI 0.766-4.437, P=0.173) (Table 5).
This study was a prospective nation-wide study that was conducted to evaluate the antibiotic resistance of E. coli isolates from Korean AUC patients (2). We applied strict criteria for selecting the isolates. The exclusion criteria were symptoms of or predisposing factors for complicated urinary tract infections such as pregnancy, symptoms lasting longer than 7 days, fever, known urological or nephrological problems, and more than 4 recurrences in the previous year (2).
The previous data has shown that among human clinical E. coli isolates, resistance to ciprofloxacin is associated with decreased virulence and phylogenetic groups B2 (3-8). If this is true, the E. coli isolates in areas with a higher prevalence of ciprofloxacin resistance should show a lower incidence of virulence factors.
It is known that the expressions of VFs and phylotypes are different between nearby or remote countries (9, 10, 12). The fluoroquinolone resistance by E. coli is highly detected mostly in the Asia-Pacific area. Moreover, ciprofloxacin should be reconsidered in some Asian countries as a first line treatment for recurrent cystitis (2, 13, 14). However, there is little data about the expressions of VFs and the phylotypes in E. coli isolates from Asian patients with AUC.
fimA was found in about 96% of the E. coli strains in our study. The high prevalence of fimA is in accordance with the studies conducted by other investigators (4, 15). Our results showed that the proportions of fimA among the ciprofloxacin resistant isolates and the ciprofloxacin sensitive isolates were not different.
P-fimbriation is a characteristic of the strains that cause upper urinary tract infections. Each P-fimbriation is composed of a major subunit papA and minor adherence-related fimbrial subunits such as papE, papF, and papG. These fimbrial proteins are encoded by a chromosomal multicistronic gene cluster termed pap. We amplified the partial gene sequence of papE and papF because it is well known that the results of gene sequencing papEF are very similar or identical to those shown for other pap families for ciprofloxacin resistance (8). The expression of papEF was significantly more prevalent in the ciprofloxacin sensitive isolates. In addition, the expression of papEF significantly reduced the ciprofloxacin resistance risk (OR 0.40, 95% CI 0.16-0.96 for papEF). The result of our study on the papEF expression was well matched with other previous studies (6, 7).
The Prs (pap-related substance) adhesin variant, which is also known as Class III G adhesin or the papG variant III, is usually the predominant papG variant among the E. coli isolates from women and children with cystitis (16). Even though the protein sequences between papG and papGIII were very similar (94% match in protein sequences with the BLAST analysis), there was no correlation between the expressions of papEF and papGIII (P=0.199). In addition, the expression of papGIII in our study was not associated with ciprofloxacin resistance. The relative high expression of papGIII (67.8%) in our study is noteworthy. Ruiz et al. (17) reported that the expression of papGIII was lower among isolates that caused cystitis than our study. We do not know by what mechanisms the difference was caused. But a geographically uneven distribution of papGIII or the differences of the patients' disease status may have contributed to the dissimilarity of papIII expression. In this respect, there was a significant correlation in our study between the presence of the papGIII gene and the presence of genes encoding cnf1 and hylA (P=0.000, P=0.006, respectively). Our results were very similar with the report of Ruiz et al. (17) reporting that there was a 100% association between the presence of the papGIII gene and the presence of alpha-hemolysin and necrotizing factor type 1.
cnf1 (cytotoxic necrotizing factor type 1) belongs to a group of bacterial necrotic substances that destroy host cells. E. coli also produce hemolysins, which are cytotoxic due to the formation of transmembranous pores in host cell membranes (18). In addition to lysing erythrocytes, hemolysin is toxic to a range of host cells in ways that probably contribute to inflammation, tissue injury and impaired host defenses. Our results showed that the genes encoding hemolysin and cnf1 among the CFSE strains were more prevalent than the CFRE strains.
Afa gene clusters encoding a-fimbrial adhesion are frequently found in E. coli causing intestinal and urinary tract infections. Members of the S-fimbrial family of adhesins are frequently expressed in the extraintestinal E. coli strains isolated from men (3). This adhesion family consists of S-fimbriae (Sfa), with its subtypes SfaI and SfaII. In our study, the dafaBC and sfaI gene did not exhibit any trend for virulence-associated ciprofloxacin resistance.
Interestingly, the incidences of virulence factors were high when compared with those of other studies (6, 7, 9). This finding is not similar with the rule "quinolone-resistant uropathogenic E. coli are less virulent". This dissimilar result may not be derived from technical incompetence. Many studies have used the multiplex PCR for determining the expression of virulence factors (15, 17), which was sometimes difficult to define a specific band according to each virulence factor. Practically, some studies have used a multiplex PCR first and then hybridized amplified products with a specific sequence product in order to obtain high specificity (8, 19). In our study, we did not use multiplex PCR because of the presence of non-specific bands and the small differences among the specific bands (for example papEF, sfaI, and fimA in Fig. 1). We did a single test "one PCR process for one virulence factor". Although time consuming, this test is easy and accurate (6). We duplicated the experiment using 20% of the samples.
In the present work, 109 E. coli isolates were classified into 4 phylogenetic groups via polymerase chain reaction: 63 group B2, 17 group A, 28 group D and 1 group B1. Of 63 group B2 isolates, 38 revealed ciprofloxacin sensitivity, whereas 25 revealed ciprofloxacin resistance. Of 17 group A isolates, 8 revealed ciprofloxacin sensitivity, while 9 revealed ciprofloxacin resistance. Of 28 group D isolates, 11 revealed ciprofloxacin sensitivity, whereas 17 revealed ciprofloxacin resistance. One isolate of group B1 sample revealed ciprofloxacin resistance. Among the ciprofloxacin resistant E. coli isolates, the shift toward the non-B2 phylogenetic groups (notably, groups A, D and B1) was noted.
Local inflammatory manifestations such as dysuria, frequency, lower abdominal pain together and pyuria are noted in the cases with AUC. Some AUC patients have a tendency of recurrent cystitis. Few studies have yet examined the expression of virulence factors and the characteristics of patients with AUC. We examined the associations between VFs and the patient characteristics as age, concomitant pyuria and a previous cystitis history within the recent year. Pyuria and a previous cystitis history were related neither with the phylotyping result nor with the VFs. The expressions of the phylotype group B2, papEF and fimA were found to be associated with a lower absolute patient age. Ciprofloxacin resistance was not related with the patients' ages in our study (Table 4). Unlike our study result, it is well known that an old age group above 65 yr showed a tendency for antibiotic resistance as well as a tendency of asymptomatic or less symptomatic infections (20, 21). The difference may be a result of different age composition and a strict guideline to exclude the complicated UTI in this study.
In conclusion, the ciprofloxacin resistant E. coli isolates from patients with acute uncomplicated cystitis exhibit the non-B2 phylotype and a selective loss of virulence factors in Korea.
Figures and Tables
 | Fig. 1Examples of single PCR for detecting virulence factors (A) and multiplex PCR for phylotyping (B).
M, 100 bp marker.
|
Table 2
The expression of virulence factors and phylotyping results in the ciprofloxacin sensitive and resistant E. coli strains that caused acute uncomplicated cystitis
ACKNOWLEDGMENTS
We would like to thank Seoul Clinical Laboratories (Seoul, Korea) for contributing valuable data and samples.
References
1. Park J, Min K, Kang D. The efficacy and safety of a once-daily extended-release ciprofloxacin tablet for the empirical treatment of symptomatic uncomplicated cystitis in Korean women. Korean J Urol. 2007. 48:35–39.

2. Kim ME, Ha US, Cho YH. Prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2006. Int J Antimicrob Agents. 2008. 31:Suppl 1. S15–S18.

3. Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991. 4:80–128.

4. Vila J, Simon K, Ruiz J, Horcajada JP, Velasco M, Barranco M, Moreno A, Mensa J. Are quinolone-resistant uropathogenic Escherichia coli less virulent? J Infect Dis. 2002. 186:1039–1042.
5. Soto SM, Jimenez de Anta MT, Vila J. Quinolones induce partial or total loss of pathogenicity islands in uropathogenic Escherichia coli by SOS-dependent or -independent pathways, respectively. Antimicrob Agents Chemother. 2006. 50:649–653.

6. Piatti G, Mannini A, Balistreri M, Schito AM. Virulence factors in urinary Escherichia coli strains: phylogenetic background and quinolone and fluoroquinolone resistance. J Clin Microbiol. 2008. 46:480–487.

7. Johnson JR, Kuskowski MA, O'bryan TT, Colodner R, Raz R. Virulence genotype and phylogenetic origin in relation to antibiotic resistance profile among Escherichia coli urine sample isolates from Israeli women with acute uncomplicated cystitis. Antimicrob Agents Chemother. 2005. 49:26–31.

8. Horcajada JP, Soto S, Gajewski A, Smithson A, Jiménez de Anta MT, Mensa J, Vila J, Johnson JR. Quinolone-resistant uropathogenic Escherichia coli strains from phylogenetic group B2 have fewer virulence factors than their susceptible counterparts. J Clin Microbiol. 2005. 43:2962–2964.

9. Grude N, Potaturkina-Nesterova NI, Jenkins A, Strand L, Nowrouzian FL, Nyhus J, Kristiansen BE. A comparison of phylogenetic group, virulence factors and antibiotic resistance in Russian and Norwegian isolates of Escherichia coli from urinary tract infection. Clin Microbiol Infect. 2007. 13:208–211.

10. Katouli M, Brauner A, Haghighi LK, Kaijser B, Muratov V, Möollby R. Virulence characteristics of Escherichia coli strains causing acute cystitis in young adults in Iran. J Infect. 2005. 50:312–321.

11. Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000. 66:4555–4558.
12. Freitag T, Squires RA, Schmid J, Elliott J. Feline uropathogenic Escherichia coli from Great Britain and New Zealand have dissimilar virulence factor genotypes. Vet Microbiol. 2005. 106:79–86.

13. Lee SJ, Cho YH, Kim BW, Lee JG, Jung SI, Lee SD, Lee SE, Kim ME, Choi YD, Rim JS, Sim BS, Cho IR, Ryu SB, Kim CS, Kim WJ, Lee TY. A multicenter study of antimicrobial susceptibility of uropathogens causing acute uncomplicated cystitis in woman. Korean J Urol. 2003. 44:697–701.
14. Ling TK, Xiong J, Yu Y, Lee CC, Ye H, Hawkey PM. Multicenter antimicrobial susceptibility survey of gram-negative bacteria isolated from patients with community-acquired infections in the People's Republic of China. Antimicrob Agents Chemother. 2006. 50:374–378.

15. Takahashi A, Kanamaru S, Kurazono H, Kunishima Y, Tsukamoto T, Ogawa O, Yamamoto S. Escherichia coli isolates associated with uncomplicated and complicated cystitis and asymptomatic bacteriuria possess similar phylogenies, virulence genes, and O-serogroup profiles. J Clin Microbiol. 2006. 44:4589–4592.

16. Johnson JR, Johnson CE, Maslow JN. Clinical and bacteriologic correlates of the papG alleles among Escherichia coli strains from children with acute cystitis. Pediatr Infect Dis J. 1999. 18:446–451.

17. Ruiz J, Simon K, Horcajada JP, Velasco M, Barranco M, Roig G, Moreno-Martínez A, Martínez JA, Jiménez de Anta T, Mensa J, Vila J. Differences in virulence factors among clinical isolates of Escherichia coli causing cystitis and pyelonephritis in women and prostatitis in men. J Clin Microbiol. 2002. 40:4445–4449.

18. Kouokam JC, Wai SN, Fällman M, Dobrindt U, Hacker J, Uhlin BE. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect Immun. 2006. 74:2022–2030.

19. Johnson JR, Delavari P, Kuskowski M, Stell AL. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis. 2001. 183:78–88.
20. Gobernado M, Valdés L, Alós JI, Garcia-Rey C, Dal-Ré R, García-de-Lomas J. Spanish Surveillance Group for E. coli Urinary Pathogens. Quinolone resistance in female outpatient urinary tract isolates of Escherichia coli: age-related differences. Rev Esp Quimioter. 2007. 20:206–210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download