Abstract
A visible cutaneous scar develops from the excess formation of immature collagen in response to an inflammatory reaction. This study examined the role of epidermal growth factor (EGF) in the formation of cutaneous scars. Twenty Crl:CD-1 (ICR) mice were used and 2 full-thickness skin wounds were made on the dorsum of each mouse. One of the wounds was treated with recombinant human EGF by local application and the other was treated with saline for control until complete healing was achieved. The EGF-treated group's wounds healed faster than the control group's. The width of the scar was smaller by 30% and the area was smaller by 26% in the EGF-treated group. Inflammatory cell numbers were significantly lower in the EGF-treated group. The expression of transforming growth factor (TGF)-β1 in the EGF-treated group was increased. It was observed that the amount of collagen in the EGF-treated group was larger than the control group. In the EGF-treated group, the visible external scars were less noticeable than that in the control group. These results suggest that EGF can reduce cutaneous scars by suppressing inflammatory reactions, decreasing expression of TGF-β1, and mediating the formation of collagen.
Scar formation is the final result in wound healing and collagen is one of the key factors in scar formation. A cutaneous scar caused by excessive inflammation and immature collagen has a distinctly different collagen pattern compared to normal skin collagen. The pattern of collagen in a scar shows densely packed fibers instead of the reticular pattern seen in unwounded dermis. Scar remodeling occurs during months to years. The extracellular matrix is dynamic and constantly being remodeled, and can be conceptualized as the balance between synthesis, deposition, and degradation (1). A scar connects the space between damaged tissue but maximum tensile strength reaches only 70% of unwounded skin (2). Although a scar is an essential part of the natural wound healing process, its apparent results are cosmetically and sometimes even psychologically troublesome.
A scar is an important issue to Asians because Asians have darker and more sensitive skin (Fitzpatrick skin type III-V) than Caucasians, resulting in more visible scar formation. To lower the chances of a visible scar, a more delicate and refined technique was applied to Asian patients in the operative aspect but the results were found to be beyond our control.
Scarring is a necessary part of wound healing but scarless wound healing can occur in fetal wounds with true tissue regeneration. Previous studies have shown that scarless fetal wound healing is dependent on both wound size and gestational age (3). In general, a wound created early in gestation heals without scarring whereas a wound created after the transition period heals similarly to postnatal wounds with scar formation (4, 5). In the fetal rat model, wound healing and regeneration of the skin and its appendage occurs without the increase of excessive immature collagen during early pregnancy or mid-term pregnancy (days 16.5-18.5). During that time, inflammatory reactions are hardly observed (4).
Lorenz et al. (6) demonstrated that intrauterine environment was not absolutely necessary for scarless healing. Scientists began to focus on intrinsic differences in response to injury by adult and fetal cells. Subsequent work has focused primarily on intercellular signaling through cytokines and growth factors. In the early period of adult wound healing, factors such as transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) are reported to be extensively involved (7-9). Special attention was focused when a study by Pedal et al. (10) showed that EGF was involved in scarless wound healing in which increased expression of EGF in the early murine fetal period was found and decreased EGF expression was noted during the scar formation at late pregnancy.
Satici et al. (11) studied the levels of EGF, TNF-α, and TGF-β concentration according to the type of scar in trachoma corneitis patients. They found that the concentration of EGF was inversely proportional to the severity of the scar. Several studies on the suppression of scar formation by different cytokines or drugs have been reported, but we have not been able to find a study showing the direct relationship of EGF to scar formation during wound healing (12-14). To support our hypothesis that EGF may reduce scar formation during the healing process, we examined the effects of EGF on scar formation through gross, histological, and immunohistological evaluations. And we also assessed the visible scar using scar assessment scale.
The Yonsei University Institutional Animal Care and Use Committee approved all animal experiment protocols. All animals received care in compliance with the National Research Council's criteria as outlined in the "Guide for the Care of Laboratory Animals" prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health.
Twenty Crl:CD-1 (ICR) mice were used in this experiment. All animals were 6-8 weeks of age and weighed approximately 30-40 g. Intraperitoneal injections of 45 mg/kg of ketamine (Ketara®, Yoohan Medica Corporation, Seoul, Korea) and 3 mg/kg of xylazine (Rompun®, Bayer HealthCare, Leverkusen, Germany) were administrated for anesthesia. The dorsal hairs were completely removed and sterilized with betadine and alcohol. Using an 8-mm punch, full-thickness skin defect wounds were created vertically in 2 areas. Subsequently, occlusive dressings were performed with Tegaderm® (3 M corporate, St. Paul, MN, USA). The following control and experimental groups were designed; the experimental group had distal wound treated using 0.2 mL (100 µg) of recombinant human EGF (Easyef®, 50 mg/100 mL ([60,000,000 IU], Daewoong Pharmaceutical, Seoul, Korea) and the control group had proximal wound treated using 0.2 mL of saline. Both wound groups were treated daily until complete wound closure.
Digital images of the dorsal wounds were taken every 3 days until complete wound closure (n=10). The external wound area of both the experiment and control groups were measured using Scion Image® (NIH-Scion Corporation, Frederick, MD, USA) software. The correlation of the time to complete wound closure and to the time to half size of the initial wound (HT50: healing time of 50% of the area) were obtained and compared objectively.
Four weeks after the creation of the wounds, full-thickness tissues biopsies were performed, including the scar and peripheral normal skin (n=10). They were embedded in paraffin and 5 µm sections were obtained and stained with hematoxylin and eosin (H&E). After being stained with H&E, tissue sections at the central scar area were observed under a microscope (BX-41, Olympus, Tokyo, Japan) at a fixed magnification (×40), and the images were digitally recorded into a computer by the OLYSIA® (Olympus, Tokyo, Japan). The scar width was determined by measuring the distance between two wound edges located at the middle point between the epidermis and the panniculus muscle. The relative area between two wound edges beneath the epidermis and above the panniculus muscle was also measured and recorded using same slide. The measurements of both groups were compared and statistically analyzed.
Three days after the creation of the wounds, full-thickness skin biopsies were obtained (n=5) in the proximal, distal, and left, right wound margins. The high power field (×400) under the magnification by light microscope was divided into 4 areas and the number of inflammatory cells in each area was counted and the mean and standard deviation of the EGF-treated and control groups were calculated and statistically compared.
Four days after the creation of the wounds, immunohistochemical staining for TGF-β1 was performed (n=5). The biopsy tissues were embedded in paraffin block and it was sectioned as 5 µm thickness. After rehydration, they were incubated with the primary antibody monoclonal rat anti-mouse TGF-β1 (TGF-β1(v):sc146: Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted to 1:100 for 1 hr. The biopsy tissues were subsequently incubated with secondary antibody tagging in brown, treated with hematoxylin as counter-staining and examined under a light microscope.
Trichrome staining was performed to stain collagen fiber. After rehydration and preheating of tissue slide, they were incubated in Bouin's solution for 15 min and cooled for 2 min. The samples were stained with Weigert's iron hematoxylin solution for 5 min, washed, and stained with Beibrich scarlet-acid fuchsin for 5 min. After 5 min incuation in phosphotungstic/phosphomolybdic acid solution, they were stained with aniline blue solution. After incubated with 1% acetic acid for 2 min, they were dehydrated and mounted.
Statistical analysis was performed using SPSS version 11 statistical software. Each measurement is shown as mean±standard deviation. All pairwise differences between the measurements of two groups were examined by a paired t-test. A P value of less than 0.05 was considered statistically significant.
The initial wound measurement after using an 8-mm punch (51 mm2) showed an area slightly larger (average, 71 mm2) compared to that of the punch size. This is due to the regional skin contracture and the centrifugal force around the wound. The wound healing rate showed a difference in time, particularly between the 3rd and 6th day. Reduction of the wound area in the experimental group was much faster than the control group (Fig. 1). The average healing time of HT50 of the EGF-treated group was 5.4 days and control group was 7.5 days. The EGF-treated group showed faster wound healing than the control group (P<0.001) (Fig. 1).
The scar width of the EGF-treated group was statistically narrower with an average of 2.21±0.54 mm compared to the control group of 3.18±0.57 mm. The EGF-treated group had a smaller scar width than the control group by an approximate average of 69.1%, which reveals that the EGF-treated group contributed to the reduction of scar width by about 30%, and this is statistically significant (P=0.025) (Figs. 2, 3). The scar area of the EGF-treated group on average was 1.54±0.25 mm2 and the control group was 2.08±0.34 mm2. This showed that the cross-sectional scar area of the EGF-treated group was approximately 74% of the control group and in the EGF-treated group, it contributed to the reduction of the scar area by approximately 26% and was statistically significant (P=0.022).
In the EGF-treated group, the number of inflammatory cells was decreased noticeably. The results showed that the mean value of the EGF-treated group was 32.6±8.8 cells and the control group was 52.4±16.2 cells. This result was also statistically significant (P=0.001) (Fig. 4).
In the EGF-treated group, TGF-β1 expression was relatively decreased compared to the control group. The control group showed increased brown secondary antibody, especially around the stroma. This finding was in contrast to the normal tissues in the vicinity of the wound where the pattern of expression of TGF-β1 was not increased in both the EGF-treated and control groups (Fig. 5).
In tissue samples examined after 4 weeks, the amount of collagen in the EGF-treated group was higher than the control group. However, regarding its arrangement and amount, it was not different from normal skin. In the control group, the pattern of the formation of immature collagen was detected but not substantial (Fig. 6).
EGF has been known to be one of the important growth factors in wound healing (9). It was discovered accidentally during the separation of the neuronal growth factor from the murine submandibular gland by Stanley Cohen in 1960 (15). It has been reported to accelerate wound healing in the skin, cornea, and gastric mucosa. EGF has been found to be similar to polypeptides such as TGF-α, heparin-binding EGF, amphiregulin, epiregulin, and betacellulin. It is secreted from platelets, keratinocytes, and macrophages that appear during the wound healing process. EGF stimulates mitosis and chemostasis of epithelial cells, consequently stimulating epithelization (16, 17). In addition, EGF has been known to influence wound healing by accelerating fibronectin synthesis (18). Clinical application has been reported to support the many roles of EGF accelerating wound healing, such as partial-thickness wounds, or chronic wounds, such as diabetic foot (19, 20).
Since 1983, recombinant human EGF (rh-EGF) has been produced in large quantities by the recombination of the human EGF gene and expression in microorganisms. It has been reported that rh-EGF stimulates cell proliferation and division by binding to EGF receptors and activating tyrosine kinase present in the cell membrane. Studies that examined growth factors involved in scar formation used 16-day-old fetus models during murine pregnancy when scarring was absent. If artificial wounds were formed on the fetus models prior to 16 days, a scar will not form because the distribution of growth factors changed around 19 days. Dang et al. (2) have reported that during this period, TGF-β1 was significantly reduced compared to the scar formation period on the 19th day and another growth factor, fibroblast growth factor (FGF-7, 10), was also decreased. Peled et al. (10) have reported that during the same period, TGF-β1 expression level was decreased and EGF was simultaneously increased. Shah et al. (7) have reported that scar formation could be suppressed by the suppression of TGF-β1,2.
In our study, to rule out the individual variation of mice, equal-sized wounds were created on each mouse. Prior to the objective evaluation of scar formation, basic wound healing levels were evaluated to confirm that EGF accelerates wound healing in various types of wounds. This was basically a reconfirmation of previous reports on enhanced epithelial healing of skin wounds of pigs and rats (9). We have also observed acceleration of wound healing by the application of EGF to chronic ulcerous wounds and the donor area of partial-thickness skin. This is due to the reaction of EGF stimulating the division of keratinocytes and fibroblasts, resulting in epithelization. In addition, EGF has been shown to stimulate the formation of collagen fibers and increase tension after skin incision wounds in mice (1, 2). Our study revealed similar findings in which EGF-treated groups achieved statistically faster reductions in wound areas where the HT50 of the EGF-treatment group was approximately 5.4 days compared to 7.5 days in the control group. Although, our study showed the effect of EGF on wound healing & scar formation, one limitation of the experimental design of this study should be addressed regarding the application of EGF. To maintain the effect of EGF continuously and mimic the physiology of growth factors behind wound healing, EGF was applied daily. However, the half life of recombinant EGF used in this study is 2.4 hr (noticed by manufacturer). Therefore, to maintain the effect of EGF during the wound healing process, EGF should be used more than single application considering half life of EGF. If that, the results of this study would be more distinct.
We harvested the tissue samples 4 weeks after wounding when they were completely healed to evaluate the scar width and area and to assess the visible scar. In order to accurately measure the width and area of the scars, it would have been ideal to evaluate the wound after a longer period but such follow-up observations could not be performed readily in mice due to their short lifespan. Another limitation in the mouse wound model is that visible scars do not last. Despite the limitations, the amount of early formed scars was thought to be significant enough at 4 weeks when the tissue samples were obtained. The measurements of the scar width and area showed that scars in the EGF-treated group were 69.1% of the control group's in regard to the width and 74% of the control group's in regard to the area. From this observation, it was possible to assume that EGF mediated an effect on the reduction of scar formation. It is without question that multiple factors are involved in reducing scar width and area. Among them is the role of EGF to enhance epithelialization but the key factor behind contraction is caused by myofibroblasts. It has been reported such myoblasts have been shown to be influenced by growth factors from adjacent cells (21). Baek et al. (22) confirmed the increase of myofibroblasts in the EGF-treated group, and based on this, it can be said that EGF mediates an effect on wound contractility through the stimulation of myoblast proliferation. Therefore, it can be concluded that EGF reduces scar width and area not only through rapid epithelization but also by contracting the wound.
One of the factors that also play a major role in scar formation is development of excessive inflammatory reaction during the wound healing process in which fibrosis occurs in abundance, leading to increased scar formation. In our study, the distribution and number of inflammatory cells in the early inflammatory reaction period in the EGF-treated group was reduced in comparison with the control group. Babül et al. (23) have reported that EGF was administered to artificial wounds of mice, and the results showed that histamine contents were decreased and the abnormal increase of collagen could be suppressed. The number of inflammatory cells was decreased in our study, which could be understood as the reduction of inflammatory reaction due to the decrease of inflammatory cytokines.
In tissues examined on the 4th week, the EGF-treated group demonstrated increased amount of collagen compared to the control group but regarding the arrangement or the amount, it was not statistically different from that of the normal skin taken around the wound. The control group showed patterns of immature collagen not seen from the groups treated with EGF or the normal skin but the amount was not substantial. Therefore, it can be reported that EGF stimulates collagen production but produces mature collagen, and that the production in quantity is similar as seen in normal skin. But to further evaluate regarding collagen synthesis and remodeling, the mouse model itself has limitations. The next step will be to examine the effect in other animal models, such as pigs, that have similar healing processes as humans.
In immunohistochemical staining, the expression of TGF-β1, which has been reported to mediate scar formation, was found to be suppressed in the EGF-treated group. TGF-β is an important growth factor in cell proliferation and division as well as the control of extracellular matrix composition. It has been reported to be released by activated T lymphocytes and macrophages primarily. TGF-β has been reported to induce fibrosis by accelerating fibroblast proliferation directly and excess TGF-β1 has been reported to be a causative of diverse fibrosis diseases together with scar contracture (7). In our study, the expression of TGF-β1 within wounds was lower in the EGF-treated group. We further examined the skin around the wound and found that the level of TGF-β1 was lower than the wound in both groups. According to this result, we can acknowledge that the EGF-treated group increased the synthesis of collagen to a near-normal level and played a role in obtaining maturity level without the influence of TGF-β1.
In conclusion, the data in this study support the hypothesis that EGF enhances not only wound healing through rapid epithelialization but also plays a role in reducing scar formation in mice. The mechanism behind scar reduction can be from fast epithelization, skin contraction, suppression of inflammatory reaction, mature collagen remodeling, and suppression of TGF-β1. Although the data supports the positive role of EGF against scar formation, further studies should be needed to clarify whether EGF plays a role through regulating fibroblast, modifying epithelialization and decreased inflammatory reaction at the molecular level. And also, these effects should be evaluated in human beings.
Figures and Tables
 | Fig. 1Time-wound area curve for degree of healing in each group shows that full-thickness wounds heal more rapidly in the EGF-treated group than the control group. Healing time (HT50) was about 5.4 days in the EGF-treated group and 7.5 days in the control group. |
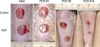 | Fig. 2Gross pictures of both wounds were taken every 3 days after full-thickness skin excision. Wound size decreases more rapidly in the EGF-treated group. POD, Post-operative day. |
 | Fig. 3Cross-sections of wounds stained with Masson's trichrome staining. Scar width and area (above the panniculus muscle) are significantly reduced in 4 weeks postoperatively in the EGF-treated group compared to the control group (×40). |
 | Fig. 4Counts of inflammatory cells. (A, B) Histopathological sections in control and EGF groups (H&E, ×100). (C) The graph shows the effect of EGF on reducing inflammatory cells (cell counts/high power field [HPF], ×400). |
 | Fig. 5Immunohistochemical observation of full-thickness wound at 4 days. The immunoactive area of TGF-β1 (brown) is markedly increased in the control group, especially in the stroma (A, C). The expression of TGF-β1 was decreased in the EGF-treated group (B, E) but increased in the adjacent normal skin area (D). |
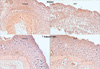 | Fig. 6Trichrome staining for collagen pattern in each group at 3 weeks. In the EGF-treated group, the epidermis is well established. Collagen bundles are mature, organized, and well arranged. The amount of collagen is similar to that of normal stroma. In contrast, an immature, disorganized collagen pattern is noted in the control group and the amount of collagen is smaller than that of normal stroma. |
ACKNOWLEDGEMENT
None of the authors have any conflict of interest in any of the products, devices, or drugs mentioned in this article.
References
1. Lorenz HP, Longaker MT. Mathes SJ, editor. Wound healing: Repair biology and wound and scar treatment. Plastic surgery. 2006. vol 1:2nd ed. Philadelphia: Saunders;209–235.
2. Dang CM, Beanes SR, Soo C, Ting K, Benhaim P, Hedrick MH, Lorenz HP. Decreased expression of fibroblast and keratinocyte growth factor isoforms and receptors during scarless repair. Plast Reconstr Surg. 2003. 111:1969–1979.

3. Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg. 1997. 32:411–415.

4. Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MW, Harrison MR. Studies in fetal wound healing: VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990. 25:63–68.

5. Lorenz HP, Whitby DJ, Longaker MT, Adzick NS. Fetal wound healing: The ontogeny of scar formation in the non-human primate. Ann Surg. 1993. 217:391–396.

6. Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS. Scarless wound repair: a human fetal skin model. Development. 1992. 114:253–259.

7. Shah M, Foreman DM, Ferguson MW. Neutrolizing antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994. 107:1137–1157.
8. Hynes JH, Johnson DE, Mast BA, Diegelmann RF, Salzberg DA, Cohen IK, Krummel TM. Platelet-derived growth factor induces fetal wound fibrosis. J Pediatr Surg. 1994. 29:1405–1408.
9. Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, Curtsinger LJ 3rd, Holtzin L, Schultz GS, Jurkiewicz MJ, Lynch JB. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989. 321:76–79.

10. Peled ZM, Rhee SJ, Hsu M, Chang J, Krummel TM, Longaker MT. The ontogeny of scarless healing II: EGF and PDGF-B gene expression in fetal rat skin and fibroblast as a function of gestational age. Ann Plast Surg. 2001. 47:417–424.
11. Satici A, Guzey M, Dogan Z, Kilic A. Relationship between tear TNF-alpha, TGF-beta1, and EGF levels and severity of cunjunctival cicatrization in patients with inactive trachoma. Ophthalmic Res. 2003. 35:301–305.
12. Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004. 123:982–989.

13. Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003. 11:25–34.

14. Liu W, Chua C, Wu X, Wand D, Ying D, Cui L, Cao Y. Inhibiting scar formation in rat wounds by adenovirus-mediated overexpression of truncated TGF-beta receptor II. Plast Reconstr Surg. 2005. 115:860–870.
15. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962. 237:1555–1562.

16. Fisher DA, Lakshmanan J. Metabolism and effects of epidermal growth factor and related growth factors in mammals. Endocr Rev. 1990. 11:418–442.

17. Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993. 165:728–737.

18. Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Epidermal growth factor induces fibronectin expression in human dermal fibroblasts via protein kinase C δ signaling pathway. J Invest Dermatol. 2004. 122:1390–1398.

19. Brown GL, Curtsinger L 3rd, Brightwell JR, Ackerman DM, Tobin GR, Polk HC Jr, George-Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986. 163:1319–1324.

20. Brown GL, Curtsinger L, Jurkiewicz MJ, Nahai F, Schultz G. Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg. 1991. 88:189–194.

21. Liu M, Warn JD, Fan Q, Smith PG. Relationships between nerves and myofibroblasts during cutaneous wound healing in the developing rat. Cell Tissue Res. 1999. 297:423–433.

22. Baek RM, Song YT, Baek SJ, Lee JH, Im TG, Yoon BH. Effects of the recombinant human epidermal growth factor on full thickness wound of the rat skin. J Korean Soc Plast Reconstr Surg. 2003. 30:201–208.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download