Abstract
Hepatic fibrogenesis, a complex process that involves a marked accumulation of extracellular matrix components, activation of cells capable of producing matrix materials, cytokine release, and tissue remodeling, is regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). The MMP-TIMP balance can regulate liver fibrogenesis. The aim of this study was to evaluate the expression patterns of MMPs and TIMPs during thioacetamide (TAA)-induced liver fibrogenesis. Chronic liver injury was induced with TAA (200 mg/kg i.p.) for 4 or 7 weeks in male Sprague-Dawley rats. Hepatic injury and fibrosis were assessed by hematoxylin-eosin (H&E) staining, and collagen deposition was confirmed by Sirius Red staining. The level of hepatic injury was quantified by serological analysis. The transcriptional and translational levels of α-smooth muscle actin (α-SMA), MMPs, and TIMPs in the liver were measured by Western blotting, RT-PCR, and immunohistochemistry. MMP, TIMP, and α-SMA were observed along fibrotic septa and portal spaces around the lobules. TAA treatment increased transcription of both MMPs and TIMPs, but only TIMPs showed increased translation. The dominant expression of TIMPs may regulate the function of MMPs to maintain liver fibrosis induced by TAA.
Liver fibrosis and end stage liver cirrhosis are worldwide healthcare problems (1). Hepatic fibrosis is a major histopathological finding associated with liver diseases due to ethanol, viral infection, cholestasis, and metabolic disorders (2). Hepatic stellate cells (HSCs) play a crucial role in liver fibrosis, including extensive remodeling of the extracellular matrix (ECM) and deposition of fibrillar collagens type I and III (3). HSCs are a major source of ECM proteins in the liver. Following liver injury, HSCs are activated and increase the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (3, 4).
MMPs, a multi-domain family of zinc-dependent endopeptidases, degrade the structural components of the ECM and many bioactive molecules (5). The MMP family is divided into secreted and membrane-anchored enzymes. MMPs are synthesized in a latent form and then converted into the extracellular active form via cleavage of specific conserved sequences.
The membrane-type MMPs (MT-MMPs) are major mediators of pericellular proteolysis. Gelatinase A, MMP-2, can degrade denatured interstitial collagens (gelatins), type V collagen, and intact type IV collagen, which are the major components of basement membranes (4, 6). MMP-2 is activated by MT1-MMP and/or plasmin (7). The mechanism of activation involves TIMP-2 interaction with MT1-MMP to form a receptor complex that regulates the activation of MMP-2 (8-11). MMP-13, the interstitial collagenase in rodents, is a specific protease capable of degrading fibrillar collagen (3).
During hepatic fibrogenesis, MMPs involved in fibrillar collagen degradation are down-regulated, whereas the expression of MMP-2 is markedly increased (12). However, this enzyme activity is regulated by powerful inhibitors, TIMP-1 and TIMP-2. TIMPs are secreted proteins that complex with MMPs to modulate activity and activation (5). Hepatic fibrosis arises when TIMPs inhibit ECM degradation excessively (13). TIMP-1 controls most MMP activity, whereas TIMP-2 inhibits MMP-2 by both inhibiting activity and preventing the formation of active enzymes (14). We examined the expression patterns of MMPs and TIMPs in thioacetamide (TAA)-induced hepatic fibrosis animal model.
The experimental protocol was reviewed and approved by the Institute Laboratory Animal Resources (ILAR) of Seoul National University. Male Sprague-Dawley rats (body weight 100±30 g) were purchased from Orient Co. Ldt. (Seongnam, Korea). Rats were housed in the animal facilities of Seoul National University College of Medicine and handled following the guidelines for the care and use of laboratory animals. Liver fibrosis was induced in rats by intraperitoneal administration of TAA (Sigma, St. Louis, MO, USA), 200 mg/kg three times weekly for 4 or 7 weeks. Four groups containing 10 rats each were treated as follows (Fig. 1): group 1, TAA for 4 weeks; group 2, saline for 4 weeks; group 3, TAA for 7 weeks; group 4, saline for 7 weeks. Blood and liver specimens were collected after sacrifice under ether anesthesia. Some liver samples were fixed in formalin for histological analysis, and some liver tissue was rapidly frozen in liquid nitrogen and stored at -80℃ for the analysis of proteins and mRNAs.
Liver tissues were fixed in 10% neutral buffered formalin solution for 24 hr and embedded in paraffin. Sections were cut to 4 µm and stained with hematoxylin-eosin. To evaluate collagen deposition, sections were stained with Sirius Red (saturated picric acid containing 0.1% Direct Red and 0.1% Fast Green FCF, Sigma). The degree of fibrosis was assessed by Scheuer's scoring system as follows: grade 0, no fibrosis; grade 1, portal fibrosis; grade 2, periportal fibrosis; grade 3, septal fibrosis; grade 4, cirrhosis (15).
Paraffin-embedded, formalin-fixed sections were baked at 65℃ for 90 min for deparaffinization, and then were rehydrated prior to antigen retrieval using a standard xylene/alcohol protocol. Endogenous peroxidase was inactivated by immersing the slides for 30 min in a hydrogen peroxide solution. After a PBS rinse, the slides were placed in protein blocking agent (Zymed, South San Francisco, CA, USA) for 30 min. The blocker was drained and the primary antibodies of MT1-MMP (Oncogene, San Diego, CA, USA), MMP-2 (Oncogene), MMP-13 (Oncogene and Chmicon, Temecula, CA, USA), TIMP-1 (Calbiochem, San Diego, CA, USA), TIMP-2 (Oncogene and Chemicon), and a-smooth muscle actin (α-SMA; Sigma, St. Louis, MO, USA) were then applied for 2 hr. Slides were rinsed in PBS and biotinylated secondary antibodies (Zymed, South San Francisco, CA, USA) were applied for 30 min. After rinsing with PBS, slides were incubated with an avidin biotin complex (Zymed) for 30 min. Slides were rinsed and incubated with DAB chromogen (Dako, Carpinteria, CA, USA) for 5 min. The reaction was stopped by rinsing in distilled water. All steps after the deparaffinization were performed at room temperature.
Frozen liver tissue was homogenized and whole proteins were extracted by ice-cold RIPA buffer (150 mM/L NaCl, 50 mM/L Tris-HCl, pH 7.4, 1 mM/L EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with cocktail protease inhibitors (Roche, Mannheim, Germany). Protein concentration was measured by the BCA method (Pierce, Rockford, IL, USA). The samples were heated at 100℃ for 8 min, and 50 µg proteins were separated on an SDS-polyacrylamide gel. After electrophoresis, the proteins were electro-transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked for 30 min with 5% skim milk (BD, Sparks, MD, USA) in TBS-T. Afterward, membranes were incubated with primary and HRP-conjugated secondary antibodies, and then incubated with an enhanced chemiluminescent ECL assay kit (Pierce, Rockford, IL, USA) according to the protocol provided by the manufacturer. Chemiluminescence was detected using Classic blue sensitive radiography film (Agfa, Belgium).
Gelatin zymography was performed by following the manufacturer's instructions (Millipore, Billerica, MA, USA). Briefly, protein extracts were prepared as above, except the heat denaturing step, and then separated by electrophoresis through a 7.5% SDS-polyacrylamide gel containing 1 mg/mL of gelatin. Gels were washed with 2.5% Triton X-100 for 30 min 2 or 3 times, followed by washing once with reaction buffer containing 50 mM/L Tris-HCl (pH 7.5), 0.2 M/L NaCl, 5 mM/L CaCl2, and 0.02% Brij 35 to remove the SDS. Gels were then incubated at 37℃ for 16 hr in reaction buffer. The gels were then stained with 0.5% Coomassie Blue R-250 (Sigma, St. Louis, MO, USA) and de-stained with an appropriate Coomassie R-250 de-staining solution (50:10:40 methanol:acetic acid:water) (16). Clear zones indicate the presence of gelatinase activity.
Total RNA was extracted from frozen tissue using a total RNA mini kit for tissue (RBC, Taipei, Taiwan), following the protocols provided by manufacturer. First strand cDNA was generated with M-MLV reverse transcriptase (Promega, Madison, WI, USA) using random primers (Promega). PCR amplification was done with rTaq (Takara, Shiga, Japan). Gene-specific primers and the sizes of the expected PCR products are listed in Table 1. Expression of β-actin was used as an internal control.
TAA treated rats showed reduced weight gain (Fig. 2A; 250.67±25.78 g vs. 402.40±29.85 g, P<0.001). The TAA treated rats also showed increased GOT and GPT levels, with greater induction at 7 weeks than at 4 weeks. Serum T-Bil and GGT were also higher, suggesting the presence of cholestatic hepatic damage. Serum albumin levels were decreased, indicating impairment of liver synthesis activity (Table 2). Grossly, TAA-treated rats showed hepatomegaly with diffuse, multi-nodular features and a hard consistency, suggesting cirrhotic changes. These features were more prominent in the TAA treated rats for 7 weeks. The liver weight/body weight ratio was significantly higher in the TAA treated group than in the untreated group (Fig. 2B). These results indicate that hepatic injury and fibrosis were successfully induced by TAA treatment.
TAA treatment for 4 weeks slightly widened the portal areas and formed thin fibrous septa throughout the hepatic parenchyma, with inflammatory cell infiltration composed of lymphocytes and plasma cells (Fig. 3A). The 7-week TAA group showed bridging or septal fibrosis connecting portal areas and central veins in a portal to portal, portal to central, and/or central to central pattern. Regenerating hepatic nodules were also observed. The amount of infiltrating inflammatory cells was also increased. Sirius red stained collagen fibers were observed in the septa (Fig. 3B). Striking collagen deposition was present in the periportal areas and areas of bridging fibrosis in the TAA treated group, whereas only mild inflammatory cell infiltration around the portal area was found in controls, with no collagen deposition or fibrous septa formation. Semi-quantification of collagen deposition in the hepatic parenchyma was 2.1±0.1 at 4 weeks and 3.7±0.5 at 7 weeks (P=0.001 and P<0.001, respectively, vs. controls), with controls scoring 0, and a higher fibrosis score after 7 weeks (P<0.001) (Fig. 3C).
We first confirmed MMP-2 expression and activity with zymography, which can discriminate between the pro and active forms of MMP-2. TAA treatment increased gelatin degradation by MMP-2 (Fig. 4A) and slightly increased MMP-2 levels (Fig. 4B). TAA slightly increased MT1-MMP and decreased MMP-13 levels, but not significantly. In contrast, TIMPs were significantly up-regulated during TAA induced fibrosis (Fig. 4B, C).
Next, we examined mRNA levels of MMPs and TIMPs with RT-PCR (Fig. 5). TAA treatment increased the expression of α-SMA, MT1-MMP, MMP-2, MMP-13, TIMP-1, and TIMP-2 and produced sharp product bands, in contrast to smeared bands in the controls. All mRNA levels did not change further between 4 and 7 weeks except TIMP-2.
Cells stained for MT1-MMP, MMP-2, TIMP-1, TIMP-2, and α-SMA were present in the portal area and around the central vein after TAA treatment (Fig. 6). Stained cells were round and scattered in mildly expanding portal areas, but changed to stellate-like spindle shapes in fully developed fibrous septa. These cells were mostly located in the interface of portal area and hepatic parenchyma and also positively stained for α-SMA, suggesting advanced fibrosis (Fig. 6). On the contrary, α-SMA positive cells were rarely identified in the control group or early stage fibrosis in the portal area. The increased cellular components in fibrous septa with progress of fibrosis had myofibroblast features. Morphologic features of epithelial cells such as hepatocytes and cholangiocytes were not observed.
TAA, a selective hepatotoxin, can induce acute and chronic hepatic injury (17-19). In the current study, we showed that TAA injection induced hepatomegaly, reduced weight gain, and produced abnormalities of serum hepatic enzymes, and a grossly cirrhotic liver. Microscopically, TAA caused changes ranging from mild portal fibrosis to fully developed cirrhotic changes. In addition to fibrosis, TAA treatment increased necro-inflammatory injuries, as shown by abnormalities of the serologic hepatic enzymes and H&E sections (Fig. 3, Table 2).
In the fibrotic liver, an imbalance occurs between excess synthesis and/or a decrease in the removal of extracellular matrix (ECM) with consequent scarring (19). MMPs and TIMPs are involved in matrix remodeling in physiological and pathological processes. MMP activities are regulated by TIMPs, which bind in substrate and tissue specific manners to MMPs, blocking their proteolytic activity (19). MMPs are synthesized and secreted, in most cases, as proenzymes that are then activated by proteinases such as plasmin (20, 21). For example, pro-MMP-2 is activated by MT1-MMP and/or plasmin, and pro-MMP-9 can be activated by MMP-3 and plasmin. Because matrix turnover is tightly regulated, activation of pro-MMPs to active MMPs is strictly controlled by complex formation with TIMPs (20). MMPs are expressed in HSCs or hepatocytes (22), whereas TIMP-1 and TIMP-2 are expressed only in HSCs (23).
Cells positive for MMPs and TIMPs in the portal areas and around the central vein also showed α-SMA expression, a surrogate marker of activated HSC (Fig. 6), in agreement with previous studies (8, 11). Therefore, HSC may provide the major sources of MMPs and TIMPs.
In addition, we investigated MMP-2 as another biomarker in the development of liver fibrosis and cirrhosis. Although we did not distinguish pro-forms and active-forms of MMP-2 in our Western blot assays, we observed that TAA increased MMP-2 activity in the zymography assay (Fig. 3), which suggests MMP-2 could participate in responses to hepatic injury or as an early reaction for liver fibrosis.
Interestingly, TAA treatment increased MMPs mRNA expression and caused sharp bands to be formed, in contrast to smeared bands present in controls (Fig. 4). Since the internal control, β-actin, is clearly detected, this result is not due to experimental errors, such as reverse transcription failures or RNA degradation. This discrepancy between mRNAs and protein levels suggest that MMPs may be altered after transcription.
Despite increased MMPs expression in TAA-induced liver fibrosis, acceleration of fibrosis was not achieved. We showed that TIMPs mRNA and protein level dramatically increase, while MMPs level increase modestly or remain relatively static as well. Thus, overexpression of TIMPs inhibits the ECM-degrading functions of MMPs may explain the establishment and maintenance of liver fibrosis. We did not characterize MMPs or TIMPs function or conversion from pro to active forms, which may be confirmed in future studies.
Differential expression of both MMPs and TIMPs occurs in fibrotic liver disease and hepatocellular carcinoma (2, 3, 11-13). The balance of MMPs and TIMPs is the key factor for liver fibrogenesis. To this respect, our results demonstrate that increased TIMP expression is more critical than MMPs in TAA-induced fibrogenesis. Understanding the balance of MMPs and TIMPs activity may identify the prevention strategies for liver fibrosis.
Figures and Tables
 | Fig. 1Experimental Design. Animals were treated weekly intraperitoneal injections of TAA at 200 mg/kg (▼), which was replaced by saline in untreated animals (▽). Total experimental period was 4 or 7 weeks. Rats were killed at the end of 4 or 7 weeks after the last injection (▲). |
 | Fig. 2Growth curves and relative liver weight in TAA-treated rats. (A) Each group (n=10) was given repeated injection with TAA or saline for 4 or 7 weeks, and body weights were measured every week. The graph shows the mean±SD of body weight. (B) Relative liver weight was calculated by the ratio to body weight. TAA treatment significantly increases the liver/body weight ratio. Data are expressed as the mean±SD, *P<0.05, TAA treated vs. untreated rats. |
 | Fig. 3Histologic features and collagen deposition of livers after 4 or 7 weeks TAA treatment. (A) TAA significantly induced hepatic fibrosis, especially at week 7 (H&E, ×100). (B) Collagen deposition (red) is prominent in TAA treated rats (Sirius Red, ×100) at week 4 and 7. (C) Fibrosis scores expressed as the average grade±SD. *P<0.001, TAA treated vs. untreated rats. |
 | Fig. 4Protein expressions of MMPs and TIMPs in TAA induced liver fibrosis. (A) MMP-2 activity is increased in TAA treated rats using zymography. (B) Protein expression pattern for MT1-MMP, MMP-2, MMP-13, TIMP-1, and TIMP-2 in TAA treated rats at week 4 and 7. (C) TIMP-1 and TIMP-2 protein levels are significantly increased in the TAA-treated group. β-tubulin was used as the internal standard. *P<0.05, TAA treated vs. untreated rat. All data are representative of three or four experiments performed with each group. |
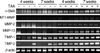 | Fig. 5Expression of α-SMA, MT1-MMP, MMP-2, MMP-13, TIMP-1, and TIMP-2 mRNA in TAA-induced liver fibrosis. TAA treatment increases mRNA level for α-SMA, MMPs, and TIMPs. RT-PCR products were obtained with specific primers for each gene and β-actin as described in Table 1. |
 | Fig. 6Immunohistochemical staining for MMPs, TIMPs, and α-SMA in TAA-treated rats. Positive cells are increased in the portal area and central vein in TAA-treated rats (×200). Black head arrows indicate positively stained cells. |
References
1. Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. J Biol Chem. 1995. 270:5331–5338.

2. Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997. 150:1647–1659.
3. Takahara T, Furui K, Yata Y, Jin B, Zhang LP, Nambu S, Sato H, Seiki M, Watanabe A. Dual expression of matrix metalloproteinase-2 and membrane-type 1 matrix metalloproteinase in fibrotic human livers. Hepatology. 1997. 26:1521–1529.
4. Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996. 271:17124–17131.
5. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schadevan-Westrum S, Crabbe T, Clements J, d'Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase "Receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998. 273:871–880.
6. Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996. 393:101–104.

7. Krane SM, Byrne MH, Lemaître V, Henriet P, Jeffrey JJ, Witter JP, Liu X, Wu H, Jaenisch R, Eeckhout Y. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996. 271:28509–28515.

8. Benyon RC, Arthur MJ. Extracellular matrix degradation and the role hepatic stellate cells. Semin Liver Dis. 2001. 21:373–384.
9. Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000. 31:75–82.

10. Lindquist JN, Parsons CJ, Stefanovic B, Brenner DA. Regulation of α1(I) collagen messenger RNA decay by interaction with αCP at the 3'-untranslated region. J Biol Chem. 2004. 279:23822–23829.
11. Nie QH, Duan GR, Luo XD, Xie YM, Luo H, Zhou YX, Pan BR. Expression of TIMP-1 and TIMP-2 in rats with hepatic fibrosis. World J Gastroenterol. 2004. 10:86–90.

12. Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006. 44:420–429.
13. Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Vidaud M, Rosenbaum J. Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of MMP expression and activity. J Hepatol. 2007. 46:69–76.

14. Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003. 327:143–147.

15. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991. 13:372–374.

16. Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990. 1:99–106.
17. Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990. 10:56–65.

18. Waters NJ, Waterfield CJ, Farrant RD, Holmes E, Nicholson JK. Metabonomic deconvolution of embedded toxicity: application to thioacetamide hepato- and nephrotoxicity. Chem Res Toxicol. 2005. 18:639–654.

19. Wang CH, Lee TH, Lu CN, Chou WY, Hung KS, Concejero AM, Jawan B. Electroporative α-MSH gene transfer attenuates thioacetamide-induced murine hepatic fibrosis by MMP and TIMP modulation. Gene Therapy. 2006. 13:1000–1009.

20. Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998. 21:75–80.
21. Cheong JY, Cho SW, Lee JA, Lee KJ, Wang HJ, Lee JE, Kim JH. Matrix metalloproteinase-3 genotypes influence recovery from hepatitis B virus infection. J Korean Med Sci. 2008. 23:61–65.

22. Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabró A, Ciancio G, Stefanni F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994. 144:528–537.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download