Abstract
To better understand the anatomic location of scalp nerves involved in various neurosurgical procedures, including awake surgery and neuropathic pain control, a total of 30 anterolateral scalp cutaneous nerves were examined in Korean adult cadavers. The dissection was performed from the distal to the proximal aspects of the nerve. Considering the external bony landmarks, each reference point was defined for all measurements. The supraorbital nerve arose from the supraorbital notch or supraorbital foramen 29 mm lateral to the midline (range, 25-33 mm) and 5 mm below the supraorbital upper margin (range, 4-6 mm). The supratrochlear nerve exited from the orbital rim 16 mm lateral to the midline (range, 12-21 mm) and 7 mm below the supraorbital upper margin (range, 6-9 mm). The zygomaticotemporal nerve pierced the deep temporalis fascia 10 mm posterior to the frontozygomatic suture (range, 7-13 mm) and 22 mm above the upper margin of the zygomatic arch (range, 15-27 mm). In addition, three types of zygomaticotemporal nerve branches were found. Considering the superficial temporal artery, the auriculotemporal nerve was mostly located superficial or posterior to the artery (80%). There were no significant differences between the right and left sides or based on gender (P>0.05). These data can be applied to many neurosurgical diagnostic or therapeutic procedures related to anterolateral scalp cutaneous nerve.
Invasive neurosurgical procedures, such as craniotomies, often cause persistent headaches (1-3). Persistent headaches are caused by neurovascular compromise, inappropriate intraneural local anesthetics, nerve traction during the surgical procedure, or compression of the nerve by scar tissue (4-6). Damage to these structures can also lead to temporary or permanent loss of sensation to the areas innervated by the nerves (7, 8). Therefore, dissection of the scalp should involve care not to induce potential damage to the cutaneous nerves.
A local anesthetic nerve block of the scalp has been shown to be the most efficient diagnostic and therapeutic tool for headaches and various neurosurgical procedures (7-9). For example, the supraorbital and supratrochlear nerves have been implicated as frontal trigger sites (4, 5), supported in part by the fact that improvement in symptoms have been demonstrated in a significant proportion of patients after chemodenervation of the corrugator supercilii muscle by botulinum toxin type A (6, 10, 11). In addition, neurosurgical interventions including awake surgery and halo vest placement require an adequate understanding of the anatomic distribution of the cutaneous nerves in the scalp in order to avoid procedure-induced pain (12, 13). There is a basis for the recurrence of peripheral neuralgia and incomplete impairment of the nociceptive conduction can occur from repeated injections, such as occurs in patients with Cushing's syndrome (14). Furthermore, inadequate nerve blocking of the scalp requires a large volume of local anesthetic solution that may induce cardiac problems and neurotoxicity (13, 15). Consequently, the nerve block of an injection point should be optimized to ensure efficient anesthesia, which requires a clear understanding of the anatomic location and distribution of the cutaneous nerves of the scalp. However, a detailed description of the anatomic location and external landmarks has rarely been studied until now.
In this study, we determined the landmarks for the localization and anatomic variation of the supraorbital, supratrochlear, zygomaticotemporal, and auriculotemporal nerves. Our data will provide important information for diagnostic and therapeutic modalities in various neurosurgical diseases and procedures which involve the anterolateral scalp cutaneous nerves.
Fifteen Korean adult cadavers were dissected in this study. None of the cadavers showed any evidence of gross pathology, previous surgical procedures, or traumatic lesions to the scalp. The age range of the cadavers was 51-82 yr (Table 1). The cadavers were fixed with 10% formalin in the Department of Anatomy of the College of Medicine of Korea University. The dissection was performed from the peripheral area of the nerve to its origin and the forehead skin was undermined. Considering the external bony landmarks which the clinicians can easily identify in the procedure, each reference point was defined for all measurements as follows: the midline and upper margin of the supraorbital rim (the uppermost line of supraorbital rim) for the supraorbital and supratrochlear nerves, the frontozygomatic suture and upper margin of the zygomatic arch for the zygomaticotemporal nerve, and the lamina trigi and upper margin of the zygomatic arch for the auriculotemporal nerve (Fig. 1). The measurements were obtained using a digital caliper (Shockproof Dial Caliper; Fred V. Fowler Inc., Newton, MA, USA). The dissection findings were recorded by a digital camera (Samsung Electronics, Suwon, Korea). Statistical analyses were processed on a personal computer with commercially available SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). Student's t-test was applied for statistical analysis. A P<0.05 was considered statistically significant.
A total of 30 anterolateral scalp cutaneous nerves were examined in this study. There was no significant difference right to left side and gender (Tables 2, 3). The supraorbital nerve arose from the supraorbital notch or supraorbital foramen 29 mm lateral to the midline (range, 25-33 mm) and 5 mm below the upper margin of the supraorbital rim (range, 4-6 mm; Figs. 1, 2A). The supratrochlear nerve exited the orbital rim located 16 mm lateral to the midline (range, 12-21 mm) and 7 mm below the upper margin of the supraorbital rim (range, 6-9 mm; Figs. 1, 2B). The zygomaticotemporal nerve pierced the deep temporalis fascia 10 mm posterior to the frontozygomatic suture (range, 7-13 mm) and 22 mm above the upper margin of the zygomatic arch (range, 15-27 mm; Fig. 3A). The zygomaticotemporal nerve was classified as one of three types based on the branch pattern (Fig. 3B). After the zygomaticotemporal nerve exited from the deep temporal fascia, the branches were usually located at a mean distance of 7.5 mm (range, 5-10 mm) from the nerve which pierced the deep temporalis fascia and 24.8 mm above the upper margin of the zygomatic arch (range, 20-29 mm). The type I pattern was the most common zygomaticotemporal nerve type and consisted of two branches in 22 cases (73%). The type II pattern was identified in 6 zygomaticotemporal nerves (20%) and the nerve was divided into three branches. In the type III pattern (2 cases), the zygomaticotemporal nerves did not have branches, rather coursed superior and posterior below the subcutaneous tissue. The auriculotemporal nerve was superficially located 4 mm anterior to the lamina trigi (range, 3-6 mm) and 13 mm along the upper margin of the zygomatic arch (range, 9-16 mm; Fig. 4A). It was classified into 3 types according to the topographic relationships between the auriculotemporal nerve and the superficial temporal artery. With respect to the superficial temporal artery, the auriculotemporal nerve usually runs posterior in 12 cases (40%) or superficially parallel in 12 cases (40%). In 6 cases (20%), the auriculotemporal nerve ran anterior to the artery (Fig. 4B).
In anatomic studies, the supraorbital nerve has been shown to originate from the frontal nerve, which is a branch of the ophthalmic nerve and is divided into the medial and lateral branches (16, 17). The medial branch has several sub-branches that perforate the frontalis muscle to innervate most scalp areas in the forehead. The lateral branch ascends between the galea aponeurotica and the pericranium to innervate a small area of the parietal scalp (6, 7, 17, 18). The supratrochlear nerve is also a branch of the frontal nerve, which itself comes from the ophthalmic division of the trigeminal cranial nerve. The supratrochlear nerve exits the supraorbital foramen, curves superior on the forehead close to the bone, and innervates partial scalp areas in the forehead (7, 17). The supratrochlear nerve is located medial to the supraorbital nerve at the supraorbital rim (17). The supratrochlear nerve shows a skewed distribution toward the medial aspect of the superior orbital rim (18). In this study, we defined the midline and upper margin of the supraorbital rim as a bony landmarks for the supraorbital and supratrochlear nerves and observed that the supraorbital nerve blocking site is located 29.04±2.34 mm lateral to the midline and 5.11±0.71 mm below the supraorbital upper margin. Considering the another landmarks that the horizontal distance is from the facial midline to the center of the foramen or notch and the vertical distance is from the superior orbital rim to the center of the foramen, the value of the supraorbital nerve blocking site is which is similar to our data (17). However, the supraorbital foramen or notch is not always palpable in the clinical setting. Therefore, we can consider the upper margin of the supraorbital rim for an alternative landmark for a supraorbital nerve block. In the case of the supratrochlear nerve, the result of measurement differed from other studies (7, 16, 18, 19). The prior study demonstrated that the supratrochlear nerve was located medial to the supraorbital nerve at the supraorbital rim an average of 9.0 mm, whereas it was located medial an average of 13.0 mm in our study (17). It is noteworthy that anatomic variations may exist in occurrence, form, and location of exits following race differences (19-23). Specifically, such an anatomic variation in the anterolateral scalp nerve has rarely been studied in the Asian population (22). For example, in a significant proportion of Chinese, the supraorbital nerve exits are located toward the medial aspect of and above the superior orbital rim compared with other data (18). These results suggest that the difference can occur between races. Therefore, the present study provides accurate information, at least in the Asian population, for the nerve structures of the anterolateral scalp region. Future studies should be focused on the ethnic variations of the locations of cutaneous nerves in the scalp.
The zygomatic nerve, the second division of the trigeminal nerve, arises in the pterygopalatine fossa and enters the orbit by means of the inferior orbital fissure. Then, the zygomatic nerve courses along the lateral wall of the orbit, and divides into two branches, the zygomaticotemporal and zygomaticofacial nerves (24, 25). The zygomaticotemporal nerve passes through the zygomaticotemporal foramen and temporalis muscle and pierces the temporal fascia about 2 cm above the zygomatic arch (24, 25), where the zygomaticotemporal nerve innervates the partial forehead and temple areas. In our data, the zygomaticotemporal nerve was usually found at a mean distance of 7.5 mm (range, 5-10 mm) from the nerve which pierced the deep temporalis fascia and 24.8 mm above the upper margin of the zygomatic arch (range, 20-29 mm). In addition, the zygomaticotemporal nerve was divided 7.5±2.31 mm from where the nerve exiting the deep temporalis fascia and consisted of three types. Therefore, we suggest anesthetic nerve injection 10-17.5 mm posterior to the frontozygomatic suture and 22-24.8 mm above the upper margin of the zygomatic arch. This is the first guideline to describe the nerve block related to the zygomaticotemporal nerve exiting from the deep temporalis fascia.
The auriculotemporal nerve originates from the posterior trunk of the mandibular nerve primarily in two branches, and the middle meningeal artery passes between the branches. This nerve runs through the deep lateral pterygoid muscle and supplies fibers to the parotid gland (26, 27). The auriculotemporal nerve is blocked at a point which is located at the level of and 10-15 mm (range, 8-20 mm) anterior to the upper origin of the helix (7). Alternatively, the auriculotemporal nerve block can be performed 4.09±0.95 mm anterior to the lamina trigi point and 13.28±2.09 mm along the zygomatic arch upper margin in our measurement. In addition, we also consider the anatomic relationship between the superficial temporal artery and the auriculotemporal nerve in the auriculotemporal nerve block. As like our data, the anesthetic block should be practiced posterior to the anatomic orientation point because the nerve almost runs superficially parallel or posterior to the artery.
In summary, we demonstrate useful landmarks for local anesthetic nerve blocks in the variable peripheral courses of the supraorbital, supratrochlear, zygomaticotemporal, and auriculotemporal nerves by performing anthropometric measurements for anterolateral scalp cutaneous nerves. These data can be applied to many neurosurgical diagnostic or therapeutic procedures related to anterolateral scalp cutaneous nerve.
Figures and Tables
Fig. 1
Schematic drawings of the reference points and bony landmarks. (A) Reference points of the supraorbital and supratrochlear nerves. a, midline; b, supraorbital upper margin. (B) Reference points of the zygomaticotemporal and auriculotemporal nerves. a, frontozygomatic suture; b, upper margin of the zygomatic arch; c, lamina trigi; d, the point from which the zygomaticotemporal nerve exits from the deep temporalis fascia; e, the auriculotemporal nerve at its exit point; line a-d, the distance from the zygomaticofrontal suture to the zygomaticotemporal nerve; line b-d, the distance from the zygomaticotemporal nerve to the zygomatic arch upper margin; line b-e, the distance from the upper margin of the zygomatic arch to the auriculotemporal nerve at its exit point; line c-e, the distance from the auriculotemporal nerve at its exit point lamina trigi. (C) Schematic drawings of the relationship between the skin area innervated by each nerve and the subcutaneous nerve location.

Fig. 2
Photographs of the supraorbital and supratrochlear nerve (A, B). Cadaveric photographs showing the supraorbital nerve (1) and supratrochlear nerve (2). Supraorbital rim upper margin was drawn as a dotted line.
M, midline; SOF, supraorbital foramen.

Fig. 3
Photograph of zygomaticotemporal nerve and schematic drawings of the zygomaticotemporal nerve branches. (A) Cadaveric photographs showing the zygomaticotemporal nerve (1) and the frontozygomatic suture (2), (B) The nerve is classified into three types depending on the branch pattern.
T, temporalis muscle.
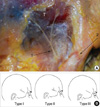
Fig. 4
Photograph of auriculotemporal nerve and schematic drawings of the relationship between the auriculotemporal nerve and the superficial temporal artery. (A) Cadaveric photographs showing the auriculotemporal nerve (1) and superficial temporal artery (2), (B) Schematic drawing of the relationship between the auriculotemporal nerve and the superficial temporal artery. Type I, The nerve runs posterior to the artery; Type II, The nerve runs anterior to artery; Type III, The nerve runs superficially parallel to the artery.
A, artery; N, nerve, Ant, anterior, Post, posterior.

References
1. De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996. 38:466–469.

3. Rocha-Filho PA, Gherpelli JL, de Siqueira JT, Rabello GD. Post-craniotomy headache: characteristics, behaviour and effect on quality of life in patients operated for treatment of supratentorial intracranial aneurysms. Cephalalgia. 2008. 28:41–48.

4. Guyuron B, Kriegler JS, Davis J, Amini SB. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005. 115:1–9.
5. Guyuron B, Varghai A, Michelow BJ, Thomas T, Davis J. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000. 106:429–434.

6. Janis JE, Ghavami A, Lemmon JA, Leedy JE, Guyuron B. The anatomy of the corrugator supercilii muscle: part II. Supraorbital nerve branching patterns. Plast Reconstr Surg. 2008. 121:233–240.

7. Andersen NB, Bovim G, Sjaastad O. The frontotemporal peripheral nerves. Topographic variations of the supraorbital, supratrochlear and auriculotemporal nerves and their possible clinical significance. Surg Radiol Anat. 2001. 23:97–104.

8. Ashkenazi A, Levin M. Three common neuralgias. How to manage trigeminal, occipital, and postherpetic pain. Postgrad Med. 2004. 116:16–18. 21–24. 31–32. passim.
10. Binder WJ, Brin MF, Blitzer A, Pogoda JM. Botulinum toxin type A (BOTOX) for treatment of migraine. Dis Mon. 2002. 48:323–335.

11. Mathew NT, Kaup AO. The use of Botulinum toxin type A in headache treatment. Curr Treat Options Neurol. 2002. 4:365–373.

12. Nguyen A, Girard F, Boudreault D, Fugere F, Ruel M, Moumdjian R, Bouthilier A, Caron JL, Bojanowski MW, Girard DC. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001. 93:1272–1276.

13. Costello TG, Cormack JR. Anaesthesia for awake craniotomy: a modern approach. J Clin Neurosci. 2004. 11:16–19.

14. Lavin PJ, Workman R. Cushing syndrome induced by serial occipital nerve blocks containing corticosteroids. Headache. 2001. 41:902–904.

15. Ohmura S, Kawada M, Ohta T, Yamamoto K, Kobayashi T. Systemic toxicity and resuscitation in bupivacaine-, levobupivacaine-, or ropivacaine-infused rats. Anesth Analg. 2001. 93:743–748.

16. Beer GM, Putz R, Mager K, Schumacher M, Keil W. Variations of the frontal exit of the supraorbital nerve: an anatomic study. Plast Reconstr Surg. 1998. 102:334–341.

17. Cuzalina AL, Holmes JD. A simple and reliable landmark for identification of the supraorbital nerve in surgery of the forehead: an in vivo anatomical study. J Oral Maxillofac Surg. 2005. 63:25–27.

18. Cheng AC, Yuen HK, Lucas PW, Lam DS, So KF. Characterization and localization of the supraorbital and frontal exits of the supraorbital nerve in Chinese: an anatomic study. Ophthal Plast Reconstr Surg. 2006. 22:209–213.

19. Saylam C, Ozer MA, Ozek C, Gurler T. Anatomical variations of the frontal and supraorbital transcranial passages. J Craniofac Surg. 2003. 14:10–12.

20. Anil A, Peker T, Turgut HB, Gulekon IN, Liman F. Variations in the anatomy of the inferior alveolar nerve. Br J Oral Maxillofac Surg. 2003. 41:236–239.
21. Agthong S, Huanmanop T, Chentanez V. Anatomical variations of the supraorbital, infraorbital, and mental foramina related to gender and side. J Oral Maxillofac Surg. 2005. 63:800–804.

22. Chung MS, Kim HJ, Kang HS, Chung IH. Locational relationship of the supraorbital notch or foramen and infraorbital and mental foramina in Koreans. Acta Anat (Basel). 1995. 154:162–166.

23. Gumusburun E, Katkici U, Erdil H, Sevim A, Gulec E. Variations of supraorbital traits. Morphologie. 2002. 86:19–22.
24. Totonchi A, Pashmini N, Guyuron B. The zygomaticotemporal branch of the trigeminal nerve: an anatomical study. Plast Reconstr Surg. 2005. 115:273–277.
25. Hwang K, Suh MS, Lee SI, Chung IH. Zygomaticotemporal nerve passage in the orbit and temporal area. J Craniofac Surg. 2004. 15:209–214.

26. De Benedittis G. Auriculotemporal syndrome (Frey's syndrome) presenting as tic douloureux. Report of two cases. J Neurosurg. 1990. 72:955–958.
27. Gulekon N, Anil A, Poyraz A, Peker T, Turgut HB, Karakose M. Variations in the anatomy of the auriculotemporal nerve. Clin Anat. 2005. 18:15–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download