Abstract
Plasmablastic lymphoma (PBL) is a recently identified entity that is considered to be a type of diffuse large B-cell lymphoma with a unique immunophenotype and a predilection for the oral cavity of patients with the human immunodeficiency virus (HIV). Although its clinical features may help in the differential diagnosis, an extraoral location in a patient without HIV makes it more difficult to suspect clinically. This case report is the first to describe a patient with PBL originating from the jejunum in a 60-yr-old, HIV-seronegative man. Computed tomography of the face, chest and abdomen showed about a 9.4×9.0 cm mass of the proximal jejunum, multiple masses in the musculoskeletal soft tissue, and multiple lymphadenopathies. The histological examinations demonstrated a large cell lymphoma with plasmablastic differentiation. The neoplastic cells were diffusely positive for MUM1, epithelial membrane antigen and lambda light chains, and focally positive for CD79a; but negative for CD3, CD20, CD30, CD34, CD45RO, CD56, CD99, and CD117. The proliferation index by Ki-67 immunohistochemistry was approximately 70%. These findings were compatible with the diagnosis of PBL. The findings in this case suggest that PBL should be included in the differential diagnosis of a small bowel mass even in a HIV-negative patient.
Plasmablastic lymphoma (PBL) is a lymphoproliferative disorder that is considered a type of diffuse large B-cell non-Hodgkin's lymphoma (NHL); it is a morphologic variant by the currently proposed World Health Organization (WHO) classification system (1). Due to its recent recognition as a unique disease entity, it has only been partially characterized, primarily on the basis of sporadic case reports (2-6). To date, PBLs have been found frequently in patients infected with the human immunodeficiency virus (HIV) (2, 3); these patients characteristically present with extranodal disease involving the oral cavity. In this report, we describe an unusual case of PBL that presented with a large mass originating from the jejunum in a HIV-seronegative patient.
A 60-yr-old man presented to the emergency department with complaints of dyspnea and dizziness. Two months ago, he underwent an upper endoscopy, colonoscopy and cardiac single photon emission computed tomography (CT) in another hospital for anemia and exertional dyspnea, and the results were all within normal limits. The patient also underwent a biopsy of a chest wall mass and a fine needle aspiration of a cervical lymphadenopathy that was incidentally detected by the CT of the chest. The results of the biopsy and fine needle aspiration were non-specific, and a follow-up appointment was made after 3 months. However, his exertional dyspnea progressively worsened over the past 1 month. On admission, he reported a weight loss of 7 kg during the last two months and melena five days ago. There were no conditions associated with immunosuppression. On physical examination, the patient was found to have a 2.5 cm ovoid mass in the submandibular area; similar but smaller lesions were present on the forehead, chest wall and right vestibule of the oral cavity. There were a right supraclavicular lymphadenopathy and bilateral axillary lymphadenopathies without tenderness.
The laboratory data on admission included a white blood cell count of 6,800/µL (72% polymorphonuclear leucocytes, 18% lymphocytes and 8% monocytes), a hemoglobin concentration of 6.2 g/dL and a platelet count of 397,000/µL. The serum iron was 13 µL/dL, total iron-binding capacity 394 µL/dL and the serum ferritin was 5.4 ng/mL. The biochemical analysis of the blood for hepatic and renal function, lactate dehydrogenase and the urine analysis were all within normal limits. The serum CEA and CA 19-9 were normal, and the tests for HIV, which were performed at admission and 3 months later, were all negative.
The conventional esophagogastroduodenoscopy showed normal esophagus, stomach and duodenum; however, a friable ulcerofungating mass was noted over 10 cm segment in the proximal jejunum (Fig. 1). The findings of multiple endoscopic biopsies revealed diffuse infiltration of neoplastic lymphoid cells with plasmablastic differentiation. CT of the abdomen showed a 9.4×9.0 cm lobulated mass with enhancement in the proximal jejunum (Fig. 2A) as well as multiple enhancing nodules in the greater omentum, transverse mesocolon, and left retroperitoneal space. The double contrast small bowel series showed a growing exophytic mass with central ulcers in the proximal jejunum (Fig. 2B), however, the other small bowel loops were normal. CT of the face and chest showed about a 2.3 cm ovoid mass in the right submandibule, multiple oval or round masses in the musculoskeletal soft tissue (sternum, costal cartilage, both ribs, clavicles, scapulas, chest wall, right sternocleidomastoid muscle, pectoralis major muscle, right supraspinatus muscle, and left retroperitoneum) and multiple lymphadenopathies (Fig. 3). Excisional biopsies of the masses from the oral cavity and the forehead and of the supraclavicular lymphadenopathy were performed, and its findings revealed a monotonous proliferation of plasmablastic cells with abundant cytoplasm and round nuclei (Fig. 4A). The neoplastic cells were diffusely positive for MUM1, epithelial membrane antigen (EMA) and lambda light chains (Fig. 4B, C) and focally positive for CD79a; however, they were negative for CD3, CD20, CD30, CD34, CD45RO, CD56, CD99, CD117, and CD138. The proliferation index with Ki-67 immunohistochemistry using the MIB-1 monoclonal antibody was approximately 70% (Fig. 4D). In situ hybridization for the Epstein-Barr virus was negative. A staging bone marrow aspirate and biopsy was free of lymphoma. The serum protein electrophoresis showed a lambda type monoclonal gammopathy. Based on these findings, the patient was diagnosed stage IV PBL.
The patient was transferred to the oncology service and treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy. There was an excellent initial response with near complete resolution of all lesions with six cycles of CHOP. Eight months later, however, the disease recurred with a newly developed mesenteric mass. Autologous peripheral blood stem cell transplantation was planned, and salvage chemotherapy was provided with etoposide, methylprednisolone, high-dose cytarabine, and cisplatin (ESHAP). However, the disease progressed and locoregional radiation therapy was given for a left forehead mass and pathologic fracture of the left clavicle. The patient is still alive for 24 months after the initial diagnosis.
PBL of the oral cavity was first described in 1997 (2); it has been recognized as a distinct entity, a subtype of diffuse large B-cell lymphoma, by the WHO classification of lymphoproliferative disorders (1). Since first described, more than 150 patients with this disease have been reported (7). PBLs have been strongly associated with immunodeficiency, most particularly in patients with the HIV infection (7). Therefore, this case is very unusual in that the PBL presented as a large jejunal mass in a HIV-seronegative patient.
The most notable feature of PBL is its predilection for the oral cavity and most patients with PBL present with primary oral lesions (2). The disease sites during progression are frequently widespread and have reportedly involved the central nervous system, abdominal viscera and musculoskeletal soft tissue (2, 7). Recently, the primary sites have been more frequently reported outside of the oral cavity or concurrent with oral involvement (4, 5). Over one-third of all cases with PBL were first noted at extra-oral locations, and the gastrointestinal tract has been observed to be the most common extraoral site (10.6%) (7). Although the clinical features of PBL, including its association with HIV, male gender and predilection for the oral cavity, may help in the differential diagnosis, cases that present with extra-oral locations and without HIV are less likely to be suspected clinically, as this case illustrates.
The histological appearance of PBL is usually monomorphic with a diffuse lymphoid infiltrate and a cohesive growth pattern. The neoplastic cells resemble plasmablasts with eccentric round or oval nuclei that have fine chromatin (2). The cytoplasm of the cells is abundant and appears deeply basophilic on Giemsa staining (2). PBL usually has a characteristic immunophenotype; it is negative for the typical B-cell antigens (e.g., CD20 and CD45) and positive for plasma cell markers such as MUM1, EMA, CD38, and CD138 (2, 7-9). PBLs invariably have restricted immunoglobulin light chains in the cytoplasm (positive staining for lambda, but not kappa), as in our case. All PBLs characteristically display a high rate of mitotic activity by the Ki-67 proliferation index (2), which is also consistent with our findings. Although not specific, the combination of these histopathological features is characteristic of PBL; therefore, immunophenotypic studies must be performed to confirm the diagnosis. PBL should be differentiated from neoplastic plasma cells and other varieties of NHL. Unlike PBL, a plasmacytoma typically consists of mature plasma cells without a high rate of mitotic activity. Diffuse large B-cell lymphomas nearly always express CD20, CD45-RA and CD79a; and Burkitt's lymphomas express CD20, CD45RA and CD79a, as well as the membrane-bound IgM heavy chain isotype, all of which are not typical for PBL (2, 7).
The general prognosis of PBL is very poor with a rapidly progressive clinical course (2, 7); half of the original 16 patients died within one year (2). An optimal chemotherapy regimen has not been established; the initial therapy has included administration of multi-agent systemic chemotherapy or, alternatively, local irradiation alone (2). Despite the advanced stage at presentation, this patient initially achieved a good response to the lymphoma-specific combination chemotherapy and is still alive for 24 months after the initial diagnosis.
In conclusion, the clinical features of PBL including its association with HIV, male gender and predilection for the oral cavity, may help in the differential diagnosis, however, an extra-oral location in a HIV-seronegative patient makes it more difficult to suspect clinically. This case highlights an unusual presentation of PBL with a large small bowel mass in a HIV-seronegative patient. Hence, PBL may be included in the differential diagnosis of a small bowel mass even in HIV-seronegative individuals.
Figures and Tables
Fig. 1
Endoscopic finding. The conventional esophagogastroduodenoscopy showed a friable ulcerofungating mass over 10 cm segment.
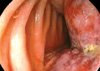
Fig. 2
Radiologic images of a jejunal mass. (A) Abdominal CT shows a 9.4×9.0 cm enhancing lobulated mass (arrow) originating from the proximal jejunum. (B) Small bowel series shows a growing exophytic mass (arrows) with central ulcers in the proximal jejunum. The adjacent bowel loops were displaced; however, the other small bowel loops were normal. A B
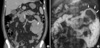
Fig. 3
CT imaging showing multiple areas of lymphoma infiltration. Arrows indicate lymphoma lesions. (A) About a 2.3 cm ovoid mass in the right submandibular space. (B, C) Multiple oval or round masses in the musculoskeletal soft tissue in the chest wall and pectoralis major muscle. (D) Chest CT shows a right supraclavicular lymphadenopathy.
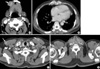
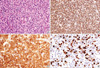
Fig. 4
Histopathology of plasmablastic lymphoma. (A) Haematoxylin and eosin section reveals a diffuse plasmablastic infiltrate with abundant cytoplasm, round nuclei, and occasionally central locating nucleoli (H&E stain, ×400). (B) The lymphoid infiltrate was positive for MUM1 (Polymer method, ×200). (C) Lambda light chain reactivity is seen in virtually all tumor cells (Polymer method, ×400). (D) The stain for Ki-67 demonstrates nuclear staining in approximately 70% of the neoplastic cells (Polymer method, ×400).
References
1. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November, 1997. Hematol J. 2000. 1:53–66.
2. Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Huhn D, Schmidt-Westhausen A, Reichart PA, Gross U, Stein H. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997. 89:1413–1420.

3. Flaitz CM, Nichols CM, Walling DM, Hicks MJ. Plasmablastic lymphoma: an HIV-associated entity with primary oral manifestations. Oral Oncol. 2002. 38:96–102.

4. Lin Y, Rodrigeus GD, Turner JF, Vasef MA. Plasmablastic lymphoma of the lung: report of a unique case and review of the literature. Arch Pathol Lab Med. 2001. 125:282–285.
5. Pruneri G, Graziadei G, Ermellino L, Baldini L, Neri A, Buffa R. Plasmablastic lymphoma of the stomach: a case report. Haematologica. 1998. 83:87–89.
6. Brown RS, Campbell C, Lishman SC, Spittle MF, Miller RF. Plasmablastic lymphoma: a new subcategory of human immunodeficiency virus-related non-Hodgkin's lymphoma. Clin Oncol (R Coll Radiol). 1998. 10:327–329.

7. Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, Redfield RR, Gilliam BL. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008. 8:261–267.

8. Colomo L, Loong F, Rives S, Pittaluga S, Martínez A, López-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004. 28:736–747.

9. Falini B, Fizzotti M, Pucciarini A, Bigerna B, Marafioti T, Gambacorta M, Pacini R, Alunni C, Natali-Tanci L, Ugolini B, Sebastiani C, Cattoretti G, Pileri S, Dalla-Favera R, Stein H. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000. 95:2084–2092.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download