Abstract
Transcatheter balloon pulmonary valvuloplasty (BPV) is considered to be the treatment of choice for neonates with critical pulmonary valvar stenosis (PVS) or pulmonary valvar atresia with intact ventricular septum accompanied by reasonable right ventricular volume. The percutaneous femoral venous access is the most preferred route for BPV in most cardiac centers. We report herein the case of a newborn baby with critical PVS with inferior vena cava interruption, severe tricuspid regurgitation and a severely enlarged right atrium. We tried BPV through the transumbilical approach with difficulty, but he was successfully treated with the assistance of a coronary artery guiding catheter.
Since the introduction of percutaneous balloon valvuloplasty for pulmonary valvar stenosis (PVS) by Kan et al. (1), balloon pulmonary valvuloplasty (BPV) has replaced surgical valvotomy for the relief of PVS throughout the world. Recently, BPV has been successfully applied in neonates with critical PVS or pulmonary valvar atresia. In most cases, successful BPV can be achieved by the percutaneous femoral venous route. However, neonates with critical PVS and inferior vena cava (IVC) interruption are more prone to undergo operation. We present herein a case of critical PVS with IVC interruption, severe tricuspid regurgitation (TR) and a severely enlarged right atrium (RA) which was successfully treated with BPV through the transumbilical venous approach. And also, we suggest pitfalls of the transumbilical approach for BPV and the usefulness of coronary artery guiding catheter from our experience.
A 1-day-old male baby was admitted to the neonatal intensive care unit for the treatment of critical PVS diagnosed in utero at 28+4 weeks of gestation. He was born by elective cesarean section at 38+6 weeks of gestation because of his mother's previous cesarean section. His birth weight was 3.38 kg. The APGAR score was 8 at 1 min and 8 at 5 min. Percutaneous O2 saturation was 70% at room air. Chest radiography showed severe cardiomegaly nearly occupying the whole chest (Fig. 1A). Transthoracic echocardiogram (TTE) revealed critical PVS with a pinhole pulmonary valvar orifice, severe TR (TR peak velocity: 4.5 m/sec), enlarged RA and a relatively small right ventricle (RV) (Fig. 2) as well as bilateral superior vena cava (SVC) with the left SVC draining into the coronary sinus and IVC interruption. The tricuspid valvar annulus measured 19 mm (Z-value >2), which indicated a good candidate for pulmonary valvotomy. We decided to perform BPV on the day of birth via the transumbilical venous approach among several venous approaches including transjugular approach.
After 5 postnatal hours, we started cardiac catheterization for BPV under mechanical ventilation and prostaglandin infusion. We initially inserted a 5F umbilical vein catheter into the umbilical vein, but the 0.018 inches short wire introduction to the RA was difficult at first. Angiography of the umbilical vein showed a stenotic or nearly closing ductus venosus (Fig. 3A). Because a 6F sheath was difficult to introduce, we inserted a 5F sheath into the inferior portion of the RA under the guidance of a 0.014 inches wire. Catheter introduction into the RV and RV outlet tract (RVOT) was extremely difficult and very different from the transfemoral approach because the posteriorly deviated sheath made the catheter easily go into the patent foramen ovale or coronary sinus (Fig. 3B). We attempted to introduce various catheters and wires into the RV. A 5F Berman catheter with and without the assistance of a curved wire could not be introduced into the RV due to severe TR, marked RA enlargement and posterior deviation of the sheath. Although a 5F Right Judkin catheter was introduced into the RV, we failed to advance it into the RVOT. A 5F Cobra catheter was also inserted into the RV, but we failed to advance it into the RVOT. A Terumo wire was not helpful in guiding the Judkin catheter into the RV. Finally, we successfully introduced a 4F Davis catheter (Terumo, Kanegawa, Japan) into the RV under the guidance of a J tip 0.035 inches wire and positioned it within the RVOT by the catheter itself. After confirming the shape of the RVOT and a small amount of forward flow to the main pulmonary artery by hand injection of contrast dye, we could successfully pass a 0.014 inches wire across the pinhole pulmonary valvar orifice. The pulmonary arterial annulus measured about 6 mm. We anchored a 0.014 inches wire at the iliac artery via the patent ductus arteriosus (PDA) and tried to balloon using a 3-mm monorail-type coronary balloon catheter (Lacrosse, Goodman, Nagoya, Japan). It was very difficult for us to push the balloon catheter through the pulmonary valve without a guiding catheter because the flexible and slippery segment of the monorail-type coronary balloon catheter, which was almost halfway out of the body, was too long to handle via the transumbilical approach. We used a 5F right coronary artery guiding catheter which created a loop within the RA (Fig. 4A) and finally succeeded in BPV using the 3- and 5-mm diameter coronary balloon catheters. We sequentially dilated the pulmonary valve with an 8-mm diameter Ultra-thin balloon catheter (Boston Scientific, Natick, MA, USA) through a 0.035 inches J-tip exchange wire in 6F Terumo hard sheath (Fig. 4B). After completion of the procedure, the peak pressure gradient between the RV and the main pulmonary artery was 18 mmHg. The ratio of peak pressure of RV to aorta was not decreased significantly because of pulmonary hypertension; from 1.33 (68/51 mmHg) to 1.27 (75/59 mmHg) after procedure. There was no acute event during the procedure. The total fluoroscopic time was 50 min and total procedure time was about 3 hr. We infused lipoprostaglandin E1 for 3 days after the procedure to increase oxygen saturation. We also supported the patient with nasal continuous positive airway pressure for 10 days because he had difficulty in breathing. Fifteen days after the procedure, the patient was discharged from the hospital without any complications. At his last follow-up at the out-patient department (9-month-old), the patient looked comfortable with 94% SaO2 and showed decreased heart size in chest radiography (Fig. 1B). The last TTE (9-month-old) revealed that the residual peak pressure gradient across pulmonary valve was 19 mmHg with moderate TR and closed PDA.
Transcatheter BPV is considered to be the treatment of choice for neonates with critical PVS or pulmonary valvar atresia with intact ventricular septum accompanied by reasonable RV volume (2). Percutaneous femoral venous access is the most preferred route for BPV at most cardiac centers. In cases where femoral venous access is absent, other access sites such as the transhepatic (3) or transjugular (4) route can be successfully used in children. In newly delivered neonates, transumbilical venous access can be another option although this route is not frequently used due to serious complications such as bacterial infection, hepatic thrombosis and hepatic necrosis. In this case, we determined to use the transumbilical venous approach first. We did not attempt the transjugular approach because we were worried about the possible RVOT mural injury during the maneuver for inserting wire and catheter through narrow infundibulum in this particular condition of the neonatal critical PS. Moreover, we could not find transjugular approach for ballooning the newborn critical PS or pulmonary atresia from the literature review. However, transjugular approach may be more beneficial than transumbilical approach in inserting the catheter into RV apex and trailing the low-profiled catheter over the securely pre-positioned wire.
We performed the transumbilical procedure early after 5 postnatal hours due to concern about the closure of the ductus venosus. Functional closure of the ductus venosus usually occurs within minutes of birth and structural closure occurs within 3 to 7 days of birth in term neonates (5). Transumbilical catheterization should be performed as early as possible for easy and safe introduction into the RA. Linde et al. (6) performed umbilical vessel catheterization in 50 neonates and were unable to reach the heart in 36 percent of the cases under the age of 6 days. They had difficulty in traversing the ductus venosus. We also had difficulty in traversing ductus venosus due to stenosis even 5 hr after birth.
Since the first report about therapeutic transumbilical balloon atrial septostomy (BAS) for complete transposition of the great arteries in 1970 (7), transumbilical BAS was suggested as an alternative method for the femoral venous access. As compared with transumbilical BAS, there have been few reports on transumbilical BPV in critical PVS or pulmonary atresia with intact ventricular septum. Posteriorly deviated course of sheath from the umbilicus to the ductus venosus and hepatic vein (Fig. 3B) is the main culprit for difficult catheter manipulations including catheter introduction into the RV and approach to the RVOT. The posteriorly deviated course of sheath used to make a soft wire and catheter coiled within the enlarged RA. In this case, a 5F right coronary artery-guiding catheter formed a loop within the RA was very helpful in introducing a coronary balloon catheter into the RVOT by preventing coiling within the RA.
In conclusion, BPV is feasible even in a neonate with IVC interruption, severe TR and severely enlarged RA via the transumbilical venous approach as one of the options for access to heart with the assistance of a coronary artery-guiding catheter.
Figures and Tables
Fig. 1
Changes in heart size before (A) and after (B) balloon pulmonary valvuloplasty. Marked cardiomegaly regressed nine months after successful valvuloplasty on chest radiography.

Fig. 2
Echocardiographic findings before balloon pulmonary valvuloplasty. Trans-thoracic echocardiograms showed a severely enlarged right atrium (RA), a relatively small right ventricle (RV) (A), a thickened tricuspid valve and a pinhole pulmonary valvar orifice with doming of leaflets (B).
PV, pulmonary valve.
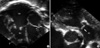
Fig. 3
Anteroposterior (A) and lateral (B) view of umbilical venous angiography. (A) Angiography of the umbilical vein (UV) showed a stenotic or nearly closing ductus venosus. (B) Catheter introduction to the right ventricular and right ventricular outlet tracts (RVOTs) was extremely difficult and very different from the transfemoral approach (thin dotted line) because posteriorly deviated sheath (thick dotted line) made the catheter easily go into patent foramen ovale (PFO) or coronary sinus (CS).

Fig. 4
Successful balloon valvuloplasty for pulmonary valvar stenosis. (A) Successful ballooning of a 3-mm diameter coronary balloon catheter with the assistance of a 5F right coronary artery guiding catheter formed a loop within the right atrium. (B) Sequential dilatation of pulmonary valve with an 8-mm diameter Ultra-thin (UT) balloon catheter.

References
1. Kan JS, White RI Jr, Mitchell SE, Gardner TJ. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med. 1982. 30:540–542.

2. Weber HS. Initial and late results after catheter intervention for neonatal critical pulmonary valve stenosis and atresia with intact ventricular septum: a technique in continual evolution. Catheter Cardiovasc Interv. 2002. 56:394–399.

3. Shim D, Lloyd TR, Cho KJ, Moorehead CP, Beekman RH 3rd. Transhepatic cardiac catheterization in children. Evaluation of efficacy and safety. Circulation. 1995. 9:1526–1530.
4. Chaara A, Zniber L, el Haitem N, Benomar M. Percutaneous balloon valvuloplasty via the right internal jugular vein for valvular pulmonic stenosis with severe right ventricular failure. Am Heart J. 1989. 117:684–685.

5. Meyer WW, Lind J. The ductus venosus and the mechanism of its closure. Arch Dis Child. 1966. 41:597–605.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download