Abstract
Most of thyroid lymphomas are B-lineage, and T-cell lymphomas are rare. Here, we report a case of primary thyroid T-cell lymphoma associated with Hashimoto's thyroiditis. A 48-yr-old woman presented with incidentally found neck mass. Histologically, the resected right lobe of the thyroid was replaced by monomorphic small atypical lymphoid cells with lymphoepithelial lesion-like change, most of which were immunoreactive for CD3, CD8, βF-1, and TIA-1. Peripheral T-cell lymphoma, unspecified, was finally diagnosed after molecular study for TCR-γ gene rearrangement. This is the second case of cytotoxic T-cell lymphoma reported in the thyroid gland so far. Unique association between thyroid follicles and neoplastic lymphocytes may be characteristic feature of this type of T-cell lymphoma.
Malignant lymphoma of the thyroid gland is rare, accounting for only 2-5% of all thyroid malignancies, and less than 2% of extranodal lymphomas (1). They are almost all B-cell lymphomas of diffuse large B-cell lymphoma and marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue (MALT) type (2). Primary thyroid T-cell lymphomas are extremely rare, and are defined as a lymphomatous process involving the thyroid gland without contiguous spread or distant metastases from other areas of involvement at diagnosis (3). To date, less than twenty cases have been described in the literature (4, 5).
Here, we present an extremely rare case of primary thyroid T-cell lymphoma of cytotoxic T-cell phenotype masquerading as lymphoepithelial lesions that are commonly found in MALT-lymphoma. To our best knowledge, only three cases showing lymphoepithelial lesions in the thyroid T-cell lymphomas have been reported in the literature (5-7).
A 48-yr-old Korean woman, presented with incidentally found neck mass in July of 2006. Her past history was non-specific. Until admission, she had been well without weight loss or hoarseness. There was no familial history of autoimmune diseases. On thyroid sonography, 3.0 cm-sized solid and cystic mass was detected in the right lobe of thyroid gland. Laboratory test showed mild leukocytosis (12,430/µL). Thyroid function tests were normal (free T4; 0.9 ng/dL, T3; 119 ng/dL, TSH; 5.16 µg/mL) with increased anti-thyroglobulin antibodies (11.25 unit/mL, reference: 0-0.3). She was serologically negative to human T-cell leukemia virus type I or human immunodeficiency virus. 99Tc nuclear medicine scans showed a large cold nodule in the right lobe. Fine needle aspiration of the thyroid was done, and it was not informative with drying artifact. Diagnostic and therapeutic right lobectomy was performed. Results of the staging work-up, which included a computed tomography scan (neck, chest and abdomen), a bone marrow examination, positron emission tomography were negative. The tumor was stage I. She received eight cycles of CHOP chemotherapy over 6 months. During the 25 months of follow up, she is alive with no recurrence.
Immunohistochemistry was done by avidin-biotin-peroxidase complex methods using antibodies against CD3 (Dako, Glostrup, Denmark, prediluted), CD20 (Dako, prediluted), CD4 (Dako, prediluted), CD8 (Dako, prediluted), T-cell-restricted intracellular antigen-1 (TIA-1, Dako, prediluted), granzyme B (Dako, prediluted), βF-1 (Dako, prediluted) and Ki-67 (Dako, prediluted). Polymerase chain reaction (PCR) for rearrangement of T-cell receptor gene (TCR)-γ was performed using formalin-fixed, paraffin-embedded specimens as described previously (8). To cover the range of the TCRγ-chain gene, the four main groups of the variable region (Vγ1-8, Vγ9, Vγ10, and Vγ11) and Jγ1/Jγ2 consensus primers were used for TCR-γ gene rearrangement amplification and PCR products were analyzed by nonradioactive single strand conformation polymorphism (SSCP).
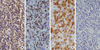
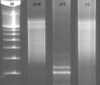
Excised specimen was composed of right lobectomy, measuring 5.3×4.0×2.0 cm, and weighing 12.7 grams. The external surface was unremarkable. The cut surface was glistening brown tan and homogeneous and ill-defined firm lesion measured 2.5×1.5×0.6 cm. Histologically, small lymphoid cells diffusely replaced the parenchyma, which were infiltrating the residual thyroid follicles with formation of lymphoepithelial lesion (Fig. 1). These cells were relatively uniform, small round cells having slightly irregular nuclei with slightly coarse chromatin and small nucleoli. Mitotic figures were occasionally seen. The surrounding atrophic thyroid epithelia showed enlarged and eosinophilic granular cytoplasm with Hürthle cell metaplasia. There were lymphoplasmacytic infiltration, lymphoid follicle formation with germinal centers, and a few eosinophils. These findings were consistent with Hashimoto's thyroiditis. The immunophenotype of atypical lymphoid cells was CD3+CD8+CD4-CD20-TIA-1+βF-1+granzymeB-CD56-CD30- (Fig. 2). Immunostain for Ki-67 proliferation index showed 1%. Above findings were strongly suggestive of T-lineage lymphoma. For definitive diagnosis, molecular study for rearrangement of TCR-γ gene was performed. SSCP showed a monoclonal rearranged band for Vγ11 gene (Fig. 3).
From the above findings, she was diagnosed as peripheral T-cell lymphoma of TCR α/β+ inactivated cytotoxic phenotype associated with Hashimoto's thyroiditis.
Initially it was difficult to recognize the present case as a T-lineage lymphoma under the light microscopy, because follicular invasion by atypical small lymphocytes, i.e. lymphoepithelial lesion, mimics MALT-type B-cell lymphoma. A lymphoepithelial lesion is a significant morphologic finding in the diagnosis of MALT-type B-cell lymphoma, however, is not specific for MALT-type B-cell lymphoma and similar intraglandular or intraepithelial invasion of small lymphocytes can be seen in Hashimoto's thyroiditis, Sjogren's disease, and enteropathy-type T-cell lymphoma (9-11).
Among thyroid T cell lymphomas reported previously, lymphoepithelial lesion-like change has been described in only 3 cases (5-7) (Table 1). Including the present case, all of them were associated with Hashimoto's thyroiditis. It is well known that MALT-type B-cell lymphoma arose in the background of chronic inflammation and autoimmune disease and some of them transformed to large B-cell lymphoma. In the thyroid gland, Hashimoto's thyroiditis markedly increases the risk of malignant lymphoma of thyroid gland and most of associated lymphomas are either MALT-type B-cell lymphoma or large B-cell lymphoma. Although there is no consensus about pathogenetic relation between Hashimoto's thyroiditis and primary thyroidal T-cell lymphoma, association of Hashimoto's thyroiditis with thyroid T-cell lymphoma in present case indicate that at least a part of thyroid T-cell lymphoma has a pathogenetic relation with Hashimoto's thyroiditis. Close association of follicular epithelial cells and neoplastic lymphocytes simulating lymphoepithelial lesion of marginal zone B-cell lymphoma may be characteristic finding of this type of T-cell lymphoma.
Because the infiltrated lymphocytes are phenotypically of helper T-cell origin with a background of Hashimoto's thyroiditis, it suggests that helper T-cell activation induced by chronic inflammation could lead to peripheral T-cell lymphoma of themselves as well as MALT-type B-cell lymphoma. However, further study may be required in CD8+ cytotoxic T-cell lymphomas arising in the background of Hashimoto's thyroiditis like the present case. These lymphoepithelial lesions in the present case are similar to those of enteropathy-type T-cell lymphoma; both individual T-lymphoma cells invade thyroid follicular epithelium or intestinal crypt epithelium. Both primary thyroid T-cell lymphoma and enteropathy-type T-cell lymphoma may be tumors of intraepithelial T-lymphocytes. Both are frequently associated with underlying autoimmune diseases such as celiac disease or Hashimoto's thyroiditis.
On the absence of a reliable immunohistochemical marker of clonality of T-lymphocytes, genetic study is the most useful method to detect the presence of a dominant T-cell clone in a lymphocytic infiltrate (2, 12). In recent days, gene rearrangement of immunoglobulins and TCR can be used as a marker of thyroid lymphoma, particularly when the histologic and immunohistochemical diagnosis of malignant lymphoma is inconclusive. The sensitivity of detecting thyroid lymphoma by the Southern blot method is about 85% in Matsuzuka's series (13), while the sensitivity by PCR is 75% in Torlakovic's series (8). In Signoretti's series, sensitivity rate was elevated up to 95% of the T-cell lymphoma using all Vγ1-8, Vγ9, Vγ10, Vγ11, and Jγ1/Jγ2 primers (14). Here, the present case was confirmed as T-cell lymphoma because it showed TCR-γ gene rearrangement, in which monoclonality was detected in Vγ11 region. This diverges from that of other conventional T-cell lymphomas which usually show monoclonalities in Vγ1-8 region.
Treatment has not yet been fully established and no survival statistics still exist for thyroid T-cell lymphoma. The patients underwent surgery, chemotherapy and radiation, combined therapy or untreated in the reports. The choice is cyclophosphamide, daunorubicin, vincristine and prednisolone (CHOP) based chemotherapy (15). Peripheral T-cell lymphoma is known to take worse prognosis than does B-cell lymphoma. However, though cases reported are rare, none of the cases of the primary thyroid T-cell lymphomas except for one died from the disease (16). Even in Okamoto's report, cytotoxic TIA-1 expressing T-cell lymphoma showed partial spontaneous regression before diagnostic hemithyroidectomy (17).
Here, we report the second case of cytotoxic T-cell lymphoma in the thyroid gland, and unique association between thyroid follicles and neoplastic T-lymphocytes, i.e. lymphoepithelial lesion, may be characteristic feature of this type of cytotoxic T-cell lymphoma.
Figures and Tables
Fig. 1
Low power view of thyroid shows diffuse effacement of normal architecture by lymphoid infiltrates (H&E. Original magnification ×100). Inset shows small atypical lymphocytes invading follicles, creating lymphoepithelial lesions (H&E. Original magnification ×400).

Fig. 2
Atypical small lymphocytes are stained with CD3 (Peroxidase. Original magnification ×400, A), CD8 (Peroxidase. Original magnification ×400, B), and cytoplasmic TIA-1-immunoreactivity (Peroxidase. Original magnification ×400, C). They are not stained with CD20 (Peroxidase. Original magnification ×400, D).
References
1. Abbondanzo S, Aozasa K, Boerner S, Thompson LDR. DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Primary lymphoma and plasmacytoma. World Health Organization classification of tumours. Pathology and genetics of tumours of endocrine organs. 2004. Lyon: IARC Press;109–111.
2. Singer JA. Primary lymphoma of the thyroid. Am Surg. 1998. 64:334–337.
3. Ansell SM, Grant CS, Habermann TM. Primary thyroid lymphoma. Semin Oncol. 1999. 26:316–323.
4. Motoi N, Ozawa Y. Malignant T-cell lymphoma of the thyroid gland associated with Hashimoto's thyroiditis. Pathol Int. 2005. 55:425–430.

5. Koida S, Tsukasaki K, Tsuchiya T, Harasawa H, Fukushima T, Yamada Y, Ohshima K, Kamihira S, Kikuchi M, Tomonaga M. Primary T-cell lymphoma of the thyroid gland with chemokine receptors of Th1 phenotype complicating autoimmune thyroiditis. Haematologica. 2007. 92:e37–e40.

6. Raftopoulos I, Vanuno D, Kouraklis G. Two unusual sites of colon cancer metastases and a rare thyroid lymphoma. Case 3. Primary T-cell lymphoma of the thyroid arising in a background of Hashimoto's thyroiditis. J Clin Oncol. 2001. 19:3576–3580.
7. Yamaguchi M, Ohno T, Kita K. gamma/delta T-cell lymphoma of the thyroid gland. N Engl J Med. 1997. 336:1391–1392.
8. Torlakovic E, Cherwitz DL, Jessurun J, Scholes J, McGlennen R. B-cell gene rearrangement in benign and malignant lymphoid proliferations of mucosa-associated lymphoid tissue and lymph nodes. Hum Pathol. 1997. 28:166–173.

9. Jaffe ES. Surgical pathology of the lymph nodes and related organs. 1995. 2nd ed. Philadelphia: W.B. Saunders Company.
10. Hyjek E, Isaacson PG. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto's thyroiditis. Hum Pathol. 1988. 19:1315–1326.

11. Isaacson PG. Knoweles DM, editor. Gastrointestinal lymphomas and lymphoid hyperplasias. Neoplastic hematology. 2001. Philadelphia: Lippincott Williams & Wilkins;1235–1261.
12. Födinger M, Buchmayer H, Schwarzinger I, Simonitsch I, Winkler K, Jäger U, Knobler R, Mannhalter C. Multiplex PCR for rapid detection of T-cell receptor-gamma chain gene rearrangements in patients with lymphoproliferative diseases. Br J Haematol. 1996. 94:136–139.

13. Matsuzuka F, Fukata S, Kuma K, Miyauchi A, Kakudo K, Sugawara M. Gene rearrangement of immunoglobulin as a marker of thyroid lymphoma. World J Surg. 1998. 22:558–561.

14. Signoretti S, Murphy M, Cangi MG, Puddu P, Kadin ME, Loda M. Detection of Clonal T-Cell Receptor gamma gene rearrangements in paraffin-embedded tissue by polymerase chain reaction and nonradioactive single-strand conformational polymorphism analysis. Am J Pathol. 1999. 154:67–75.
15. Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E, Fisher RI. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998. 339:21–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download