Abstract
Granulocyte-colony stimulating factor (G-CSF) is a naturally occurring glycoprotein that stimulates the proliferation and maturation of precursor cells in the bone marrow into fully differentiated neutrophils. Several reports of G-CSF-producing malignant tumors have been published, but scarcely any in the hepatobiliary system, such as in hepatocellular carcinoma (HCC). Here, we encountered a 69-yr-old man with a hepatic tumor who had received right hepatic resection. He showed leukocytosis of 25,450/µL along with elevated serum G-CSF. Histological examination of surgical samples demonstrated immunohistochemical staining for G-CSF, but not for G-CSF receptor. The patient survived without recurrence for four years, but ultimately passed away with multiple bone metastases. In light of the above, clinicians may consider G-CSF-producing HCC when encountering patients with leukocytosis and a hepatic tumor. More cases are needed to clarify the clinical picture of G-CSF-producing HCC.
The concept of colony stimulating factor (CSF) as a hematopoietic induction, differentiation, and growth factor was first discussed in 1966 (1). A case of malignancy was later reported with increased CSF activation in serum and urine in 1974 (2). Afterwards, it was demonstrated for the first time that CSF was directly produced in a lung cancer tissue specimen in 1977 (3).
Granulocyte-colony stimulating factor (G-CSF) is recognized as a naturally occurring glycoprotein that stimulates the proliferation and maturation of precursor cells in the bone marrow into fully differentiated neutrophils (4). Although several accounts of G-CSF-producing malignant tumors in lung cancer exist, few have been observed in the digestive system. Notably, there have been scarcely any cases found in primary liver cancer, such as hepatocellular carcinoma (HCC). Here, we present a rare case of G-CSF-producing HCC that was confirmed by immunohistochemistry.
A 69-yr-old man was admitted to our hospital in July 1999 suffering from fever, general fatigue, weight loss, and right upper abdominal pain.
On examination, the patient was 157 cm tall and weighed 43 kg. His temperature was 36.2℃. He showed no signs of alcohol addiction and had no indications of co-morbidities, such as diabetes mellitus or dyslipidemia. His family medical history was clear of any hepatic disorders. He had no signs of anemia or jaundice in conjunctiva and presented with no abdominal masses or hepatosplenomegaly, but did complain of tenderness on the right side hypochondrium. Neurological and chest examinations revealed no abnormal findings.
Laboratory tests showed a white blood cell count of 25,450/µL with 90% neutrophils, a red blood cell count of 367×104/µL, and a platelet count of 35.2×104/µL. His hemoglobin value was 10.1 g/dL and hematocrit was 31.2%. Blood chemistry showed aspartate aminotransferase of 57 U/L, alanine aminotransferase of 36 U/L, alkaline phosphatase of 949 U/L (normal range: 115 to 359 U/L), gamma-glutamyl transpeptidase of 313 U/L, cholinesterase of 44 U/L, total protein of 6.7 g/dL, and total albumin of 2.7 g/dL. C-reactive protein (CRP) was found to be 13.1 mg/dL in serological studies. The serum level of AFP was 2.0 ng/mL, the level of protein induced by vitamin K absence or antagonist II (PIVKA II) was 43 mAU/mL, the level of CEA was 9.9 ng/mL(normal value: less than 5.0 ng/mL), and the level of CA19-9 was 6.0 U/mL. Tests for hepatitis B virus surface antigen (HBsAg), hepatitis B core antibody (HBcAb), and hepatitis C virus antibody were all negative. Elevations in serum G-CSF and interleukin-6 (IL-6) were seen at 62 pg/mL (normal value: less than 18.1 pg/mL) and 26.7 pg/mL (normal value: less than 4.0 pg/mL), respectively. Bone marrow aspiration and a biopsy specimen revealed hypercellularity of mature neutrophils with normal erythropoiesis and megakaryopoiesis.
A hypoechoic tumor 5 cm in diameter was detected by ultrasonography between the anterior inferior segment (S5) and anterior superior segment (S8) of the liver. The tumor presented as a slightly low density area in pre-contrasted computed tomography (CT). It was enhanced in early phase contrast enhanced CT and accompanied with diffuse enhancement in the surrounding area, and finally washed out in the late phase with delayed hyper-enhancement in the surrounding area (Fig. 1). Magnetic resonance imaging (MRI) showed low and high intensity nodules in T1 and T2 weighted imaging with fat suppression, respectively. Angiographic examination showed that the tumor had hypervascularity. We clinically diagnosed the hepatic tumor to be common HCC according to these findings and the surrounding area to be secondary inflammatory change associated with the tumor.
We initially considered the possibility of co-infection since the patient had fever, extreme leukocytosis, and high serum levels of CRP. We intravenously administered 2 g/day sulbactam/cefoperazone and 2,400 mg/day clindamycin for 10 days, and then changed treatment to 1 g/day meropenem and 2,400 mg/day clindamycin for 9 days. No effects were seen, nor could we detect any infective foci in other organ sites by radiography or CT. Blood cultures were also tested several times after admission but were all negative for bacteria and fungus. CT images of the tumor before and after antibiotic administration did not differ. Based on the above, we concluded that the patient had no co-infections and diagnosed him as having a paraneoplastic syndrome.
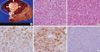
The patient received right hepatic resection in September later that year. His blood leukocyte counts decreased to normal range, and serum G-CSF and IL-6 deceased to 12 pg/mL and 5.9 pg/mL, respectively. An encapsulated gray-white nodule with foci of necrosis was seen by cut surface of the resected liver (Fig. 2A). Resected specimens of tumor histologically revealed that the tumor was a moderately differentiated hepatocellular carcinoma (Fig. 2B). A specimen of liver parenchyma adjacent to the tumor, which was diffusely enhanced by contrast enhanced CT, showed marked infiltration with neutrophils within the widened sinusoid that represented congestion (Fig. 2C). The tumor showed positive staining for hepatocyte paraffin 1 (Hep par 1) and G-CSF (Anti-G-CSF [Ab-1], mouse monoclonal antibody, Calbiochem, Darmstadt, Germany) in the cytoplasm, but was negative for G-CSF receptor (G-CSF receptor antibody [S-1284], mouse monoclonal antibody, Abcam, Cambridge, UK) (Fig. 2D-F). We thus diagnosed this tumor as a G-CSF-producing hepatocellular carcinoma.
The patient had regular follow-ups for about four years without any recurrence. He experienced rib pain in 2003 and was diagnosed as having multiple bone metastases by several imaging examinations. He was admitted to our hospital again for palliative care, and succumbed to his illness one month later. His serum G-CSF at the time of death was 18 pg/mL and within normal range.
All cases of G-CSF-producing HCC reported in English literature are listed in Table 1 (5, 6). As G-CSF-producing HCC is extremely rare, only two cases have been documented until now. Here, we present the third such case, along with immunohistochemical proof of G-CSF expression.
The following findings are indicative of G-CSF producing tumors: elevation of serum G-CSF and an increased leukocyte count, transient decreases in G-CSF and leukocyte count to normal ranges after tumor resection, a simultaneous increase in G-CSF and neutrophil count with tumor recurrence, and an elevation in G-CSF expression levels in resected specimens on the basis of immunohistochemical staining or real-time reverse transcriptase polymerase chain reaction. One direct way to prove G-CSF production on the tumor cells is by immunohistochemical techniques (7). In this case, we could clearly demonstrate that the hepatic tumor produced G-CSF by immunohistochemical analysis of specimens taken during his operation (Fig. 2E). Extreme leukocytosis and significant elevation of serum G-CSF were also noticed. Furthermore, both serum white blood cell counts and G-CSF levels decreased after resection of the tumor. In light of these, we could be sure that this case was G-CSF-producing HCC.
The prognosis of G-CSF-producing tumors is generally considered to be poor and depends largely on the primary disease (8). Such a prognosis was also noted in the previous two cases of G-CSF-producing HCC. In support of this, G-CSF was demonstrated to stimulate growth of a non hematopoietic malignant cell line in vivo (9) and is considered to be an autocrine growth factor in rapid tumor proliferation and metastasis (10-13).
We also performed immunohistochemical staining for the G-CSF receptor, which yielded negative findings. This is the first case of G-CSF-producing HCC in which immunohistochemical staining for G-CSF receptor was performed. Although the localization of the tumor and absence of liver cirrhosis in this patient may have contributed to a better prognosis, we can also speculate that the absence of G-CSF receptors in the patient's tumor caused a deficiency or absence of autocrine growth, which led to a more favorable prognosis. In a similar manner, G-CSF-receptor-positive groups of oral and mesopharyngeal squamous cell carcinomas had a significantly lower disease-free and overall survival rate than G-CSF-receptor-negative groups (14).
Another difference between our case and previous ones lies in the degree of tumor cell differentiation seen in histopathology; our case showed moderate differentiation, but the others were poorly differentiated (Table 1). It may be likely that local expression and interactions between G-CSF and G-CSF receptors induce differentiation of HCC cells into an immature phenotype.
Lastly, serum values of IL-6 were elevated both in our case and in case 2 (6). It has been reported that co-production of G-CSF and IL-6 is associated with the production of IL-1, a known as inflammatory cytokine, in G-CSF producing cancer cell lines (15). High levels of serum IL-6 and CRP in the present case may have been responsible for the chief complaint of fever. IL-6 is considered to act as an endogenous pyrogen (16, 17) that regulates the synthesis of acute phase proteins, including CRP (18, 19). However, we were unable to clarify the production of IL-6 (IL-6 [R-49L]: sc-90110, mouse monoclonal antibody, Santa Cruz Biotechnology, Inc., Santa, Cruz, CA, USA) or expression of IL-6 receptor (gp130 [AN-H2], sc-9994, mouse monoclonal antibody, Santa Cruz Biotechnology, Inc.) immunohistochemically in our case. It is possible that the antibodies used were not sensitive enough for this tumor.
In conclusion, clinicians should consider G-CSF-producing HCC when encountering patients with leukocytosis and a hepatic tumor, and radical surgery may provide a more favorable prognosis in such instances. Further cases are needed to clarify the clinical findings of G-CSF-producing HCC.
Figures and Tables
Fig. 1
Computed tomography findings. (A) The tumor measuring 5 cm in diameter between the anterior inferior segment (S5) and the anterior superior segment (S8) of the liver showed hyper-enhancement (black arrow heads) in the early phase of dynamic enhanced CT accompanied with diffuse enhancement in the surrounding area (white circle). (B) It showed complete washout (black arrow heads) in the late phase with delayed hyper-enhancement in the surrounding area (white circle).

Fig. 2
Gross and microscopic findings of the tumor. (A) Cut surface of the resected liver showed an encapsulated gray-white nodule (white arrow heads) with foci of necrosis. The area adjacent to the tumor (white circle) revealed prominent congestion. Non-neoplastic liver parenchyma was not cirrhotic. (B) Microscopic findings showed atypical cells lying in sheets with marked infiltration of neutrophils and lymphocytes, which were diagnosed as a moderately differentiated hepatocellular carcinoma (H&E, ×20 magnification of the objective lens). (C) Liver parenchyma adjacent to the tumor, diffusely enhanced by contrast enhanced CT, showed prominent congestion and marked infiltration with neutrophils within the widened sinusoid (H&E, ×20 magnification of the objective lens). The other parts of liver did not present findings of chronic hepatitis or cirrhosis (not shown). (D) The tumor lesion was stained with hepatocyte paraffin 1 (Hep par 1) (×20 magnification of the objective lens). (E) Immunohistochemical examination also showed positive staining for granulocyte-colony stimulating factor (G-CSF) in the cytoplasm of atypical cells (×20 magnification of the objective lens). (F) Immunohistochemical examination showed negative staining for G-CSF receptors in the tumor cells (×20 magnification of the objective lens).
References
1. Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966. 44:287–299.
3. Asano S, Urabe A, Okabe T, Sato N, Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977. 49:845–852.

4. Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992. 327:28–35.
5. Yamamoto S, Takashima S, Ogawa H, Kuroda T, Yamamoto M, Takeda A, Nakmaura H. Granulocyte-colony-stimulating-factor-producing hepatocellular carcinoma. J Gastroenterol. 1999. 34:640–644.

6. Araki K, Kishihara F, Takahashi K, Matsumata T, Shimura T, Suehiro T, Kuwano H. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007. 27:716–721.

7. Shimamura K, Fujimoto J, Hata J, Akatsuka A, Ueyama Y, Watanabe T, Tamaoki N. Establishment of specific monoclonal antibodies against recombinant human granulocyte colony-stimulating factor (hG-CSF) and their application for immunoperoxidase staining of paraffin-embedded sections. J Histochem Cytochem. 1990. 38:283–286.

8. Higaki I, Hirohashi K, Fukushima S, Wanibuchi H, Seike N, Yamane T, Kubo S, Tanaka H, Shuto T, Yamamoto T, Kinoshita H. Renal pelvic carcinoma producing granulocyte colony-stimulating factor: report of a case. Surg Today. 2001. 31:266–268.

9. Segawa K, Ueno Y, Kataoka T. In vivo tumor growth enhancement by granulocyte colony-stimulating factor. Jpn J Cancer Res. 1991. 82:440–447.

10. Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J, Nishi T, Amano Y. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995. 55:3438–3443.
11. Baba M, Hasegawa H, Nakayabu M, Shimizu N, Suzuki S, Kamada N, Tani K. Establishment and characteristics of a gastric cancer cell line (HuGC-OOHIRA) producing high levels of G-CSF, GM-CSF, and IL-6: the presence of autocrine growth control by G-CSF. Am J Hematol. 1995. 49:207–215.

12. Kyo S, Kanaya T, Takakura M, Inoue M. A case of cervical cancer with aggressive tumor growth: possible autocrine growth stimulation by G-CSF and Il-6. Gynecol Oncol. 2000. 78:383–387.

13. Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999. 155:1557–1567.

14. Tsuzuki H, Fujieda S, Sunaga H, Noda I, Saito H. Expression of granulocyte colony-stimulating factor receptor correlates with prognosis in oral and mesopharyngeal carcinoma. Cancer Res. 1998. 58:794–800.
15. Suzuki A, Takahashi T, Okuno Y, Tsuyuoka R, Fukumoto M, Nakamura K, Imura H. IL-1 production as a regulator of G-CSF and IL-6 production in CSF-producing cell lines. Br J Cancer. 1992. 65:515–518.

17. Luheshi GN. Cytokines and fever. Mechanisms and sites of action. Ann N Y Acad Sci. 1998. 856:83–89.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download