Abstract
We have assessed the efficacy and safety of Escherichia coli extract (ECE; Uro-Vaxom®) which contains active immunostimulating fractions, in the prophylactic treatment of chronically recurrent cystitis. Forty-two patients with more than 2 episodes of cystitis in the proceeding 6 months were treated for 3 months with one capsule daily of ECE and observed for a further 6 months. The primary efficacy criterion was the number of episodes of recurrent cystitis during the 6 months after treatment compared to those during the 6 months before treatment. At the end of the 9-month trial, 34 patients (all women) were eligible for statistical analysis. Their mean age was 56.4 yr (range, 34-75 yr), and they had experienced recurrent urinary tract infections for 7.2±5.2 yr. The number of recurrences was significantly lower during the 6-month follow-up period than during the 6 months preceding the trial (0.35 vs. 4.26, P<0.001). During the follow-up, 28 (82.4%) patients had no recurrences and 4 (11.8%) had 1 each. In patients who relapsed, ECE alleviated cystitis symptoms, including painful voiding, frequency and urgency. There were no serious adverse events related to the study drug. Our study demonstrates the efficacy and safety of ECE in the prophylactic treatment of chronically recurrent cystitis.
Urinary tract infection (UTI) is a common cause of morbidity and mortality, especially in women, with 25% to 30% of 20 to 40-yr-old women reporting a history of UTI treatment. Escherichia coli is by far the most common bacterial pathogen in UTI, accounting for more than 85% of cases of acute cystitis and pyelonephritis as well as for more than 60% of cases of recurrent cystitis (1).
The significant socioeconomic implications of UTI have generated considerable interest in the prevention of recurrences. Although long-term, low-dose antibiotic prophylaxis (e.g. trimethoprim-sulfamethoxazole) has been demonstrated to be highly effective in reducing the risk of recurrent UTI, repeated use of antibiotics has led to bacterial resistance. Other prospects for UTI prophylaxis include natural compounds, bacterial interference and immunization (2). The most commonly used natural compound appears to be cranberry juice. In a double-blind, randomized, controlled study, daily ingestion of 300 mL of cranberry juice reduced bacteriuria with pyuria but failed to decrease the incidence of symptomatic UTIs (3). The concept of bacterial interference is based on not treating asymptomatic colonization, such that "good" bacteria prevent symptomatic UTI caused by "bad" bacteria. There have been provocative data suggesting non-treatment in patient populations with a high prevalence of asymptomatic bacteriuria, including the elderly, school girls and patients with spinal cord injury (4-7), but lack of information on the strains causing asymptomatic colonization in these patient populations is a major limitation of this concept. Immunotherapy may therefore represent the most effective alternative in the prevention of recurrent UTI. For example, oral immunization with E. coli extract (ECE; Uro-Vaxom®, OM Laboratories Meyrin/Geneva, Switzerland), a combination of immunoactive fractions of E. coli strains, has gained popularity, primarily in European countries. Administration of a lyophilized extract of 18 uropathogens has been found to increase non-specific and specific humoral and cellular immune responses by stimulating the production of interferon-γ and tumor necrosis factor-γ and the activities of lymphocytes and macrophages (8-10).
Although clinical trials of ECE commenced as early as 1980 (11, 12), these studies focused on its efficacy during and for 3 months after treatment, but not for longer periods of time (13, 14). To our knowledge, there have been no clinical studies of ECE in which patients were followed for up to 6 months after treatment. We therefore assessed the efficacy and tolerability of ECE administration in the prophylactic treatment of chronically recurrent cystitis for 6 months after treatment.
Patients with more than 2 episodes of cystitis, defined as ≥105 c.f.u. bacteria/mL and white blood cell (WBC) ≥6/HPF in mid-stream urine with concomitant symptoms such as painful and irritating voiding symptoms, during the proceeding 6 months were screened, and 42 patients were enrolled in this multicenter prospective study. Patients with vesicoureteral reflux, obstructive uropathy, urinary lithiasis, renal impairment (defined as serum creatinine >2.5 mg/dL) and urologic procedures that induced UTI were excluded. The study protocol was approved by the institutional review board in each hospital, and all patients provided written informed consent before entry.
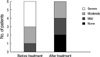
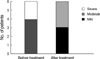
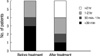
At the start of the trial, all patients were in acute recurrence and were therefore treated with antibiotics. After confirming that their urine was sterile, patients were treated for 3 months with one capsule daily of ECE, containing 6 mg of lyophilized immunostimulating fractions, and observed for an additional 6 months without treatment. UTI episodes occurring during the 6 month follow-up period were treated with pertinent antibiotics, and symptom severity was assessed. The degree of urgency was assessed according to the Indevus Urgency Severity Score (IUSS), in which 0 represents no, 1 represents mild, 2 represents moderate, and 3 represents severe urgency. Painful voiding, abdominal/flank pain and fever were each scored according to a similar scale. Frequency was assessed according to a 5 point scale, in which 0 represents >3 hr, 1 <3 hr, 2 <2 hr, 3 <1 hr, and 4 <30 min. Patients who developed UTI during ECE treatment were excluded from the study. Blood chemistry was performed at entry and after 3 months to monitor drug safety. Midstream urine culture and urinalysis were performed at study outset, and 3 and 9 months after the start of ECE treatment, and at any symptom recurrence. At each visit, patients were questioned about compliance and any adverse events.
The primary efficacy criterion was the number of episodes of recurrent cystitis which was measured with the medical record (defined by urinalysis, urine culture, and the presence of concomitant symptoms) during the 6 months after treatment compared to those during the 6 months before treatment. Secondary efficacy criteria included the severity of cystitis symptoms, including dysuria, frequency and urgency, in those who relapsed. Safety evaluations included comparisons of hepatic and renal function and adverse events before and after treatment.
For the determination of sample size, based on an estimated clinical efficacy rate for Uro-vaxom of 80.7% (α=0.05) (15), the sample size was calculated to be 27 patients. Considering an estimated 10% drop-out rate, the actual size of study population should be at least 30 patients. Quantitative data were expressed as mean±SD. Statistical analyses included parametric one-way ANOVA and nonparametric Wilcoxon's matchedpairs test. Differences were considered significant at P<0.05.
Of the 42 enrolled patients, 8 were excluded, 2 for gastrointestinal problems such as nausea and abdominal pain, 1 for failure to return after the first visit for reasons unrelated to the study medication, 3 for withdrawal of consent, and 2 who developed symptomatic UTI recurrences during ECE treatment. Accordingly, 34 patients were eligible for efficacy analysis at the end of the 9-month trial. All 42 recruited patients were included in the safety analysis. The mean age of the 34 included patients, all women, was 56.4 yr (range 34-75 yr), and they had experienced recurrent UTIs for 7.2±5.2 yr.
The number of recurrences per patient was significantly lower during the 6 months after the end of treatment than during the 6 months prior to treatment (0.35 vs. 4.26, P<0.001). During follow-up, 28 patients (82.4%) had no recurrences and 4 (11.8%) had 1 each. In addition, one patient (2.9%) had 2 recurrences and one (2.9%) had 6. Administration of ECE to the 6 patients who relapsed during follow-up alleviated cystitis symptoms, including painful voiding, frequency and urgency (Figs. 1, 2, 3). E. coli was the organism most frequently isolated from urine, both before (87%) and after (50%) treatment.
We divided the patient population into 2 subgroups: those with 'severe' and 'non-severe' cystitis, defined as ≥6 and <6 episodes, respectively, during the 6 months preceding the trial. Of the 10 patients with 'severe' cystitis, only 3 (30%) experienced recurrences, compared with 3 of 24 patients (12.5%) with 'non-severe' cystitis, a difference that was not statistically significant (P>0.05).
During treatment, 2 patients suffered from mild gastrointestinal problems: one from nausea and the other from abdominal pain, each of which lasted for 1 week. These adverse events ceased when they stopped taking the study drug, and both patients therefore quit the trial. The remaining patients did not complain of any discomfort, including skin pruritus and vertigo, which had been reported in other trials of ECE. Hepatic and renal functions, as assessed in laboratory studies, remained normal after taking ECE (Table 1).
The main therapeutic approach in UTI has been the administration of antibiotics, which is usually effective during the acute phase. For patients with chronic or recurrent UTIs, however, repetitive intake of antibiotics, even at clinically therapeutic doses, may lead to the emergence of antibiotic-resistant bacterial strains as well as impairment of the patient's natural immune defense system (16, 17). Although attempts have been made to control or reduce the frequency of acute exacerbations of UTI using natural compounds such as cranberry juice, the results have not been promising (3). In contrast, bacterial vaccines have been shown effective by improving the patient's own immune response (8-10). ECE, an immunomodulating preparation containing the lyophilized extract of 18 uropathogens, has been shown to up-regulate the activity of phagocytes, B-lymphocytes and natural killer cells (8-10, 18). Animal experiments have indicated that repeated oral administration of ECE can stimulate the formation of serum IgA and IgG in mice (19), and to activate bacterial killing by polymorphonuclear cells in rabbits, thus enhancing the clearance of bacteria from the blood stream (20).
ECE has been utilized clinically for more than 20 yr and has a good safety profile. At present, it is used to prevent recurring UTIs in children and adults (11-14, 21, 22). Many clinical trials have assessed the ability of ECE to prevent recurrent UTIs. For example, a double-blind placebo-controlled multicenter study in 166 patients with recurrent UTIs has shown that, during a 3-month observation period, patients on ECE experienced significantly fewer recurrences, less severe signs and symptoms of UTI and decreased usage of antibiotics and chemotherapeutics compared with patients taking placebo (14). In a second trial, in 112 patients with recurrent lower UTIs, patients in the ECE group (n=58) experienced 65.8% fewer episodes of UTIs than patients in the placebo group (n=54), without any critical side effects (13).
Subset analysis showed that only 30% of patients who experienced severe UTI, defined as ≥6 cystitis episodes before the trial, experienced recurrences after treatment, a frequency that did not differ significantly from the 12.5% rate observed in patients with less severe UTI (<6 episodes before treatment). The ability of this E. coli extract to prevent recurrences in more recurrent patients indicates that this agent may have greater treatment benefit in patients with more severe UTI.
Although this was not a double-blind, placebo-controlled study, a six-month period before ECE treatment was regarded as internal control to compare to 6 months after ECE treatment. To our knowledge, it is the first clinical trial to investigate the preventive efficacy of ECE for 6 months after the end of treatment. We found that ECE significantly reduced the number of recurrent episodes of UTIs 3.9-fold, as well as the severity of UTI symptoms, including urgency, painful voiding and frequency. These effects of ECE may be due to its enhancement of immune responses, leading to control of bacterial infection.
The mechanism of action of ECE is based on its ability to boost the overall immune system, not on the direct inhibition of E. coli. However, we found that ECE treatment reduced the incidence of E. coli in urine cultures, although the difference was not significant.
Over the past two decades, ECE has been widely accepted as an effective immunostimulant, with a good safety record. It was reported that most patients treated with ECE experience minor adverse events as frequently as patients in the placebo group. The most frequent adverse events were headache and gastrointestinal side effects, but there were no safety concerns with regard to laboratory variables and clinical signs (23).
Our study also confirmed the safety and efficacy of ECE during and for 6 months after the end of treatment. Repetitive use of antibiotics has been found to suppress the immune system. Patients with frequent UTI recurrences require an immunostimulating drug to prevent further depression of the immune system. The immunostimulant ECE has been shown to effectively suppress the recurrence of cystitis with resultant decrease in severity of UTI symptoms. Our study demonstrates the efficacy and safety of ECE in the prophylactic treatment of chronically recurrent cystitis.
Figures and Tables
References
3. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994. 271:751–754.

5. Hansson S, Caugant D, Jodal U, Svanborg-Edén C. Untreated asymptomatic bacteriuria in girls: I--Stability of urinary isolates. BMJ. 1989. 298:853–855.

6. Hansson S, Jodal U, Lincoln K, Svanborg-Edén C. Untreated asymptomatic bacteriuria in girls: II--Effect of phenoxymethylpenicillin and erythromycin given for intercurrent infections. BMJ. 1989. 298:856–859.

7. Sotolongo JR Jr, Koleilat N. Significance of asymptomatic bacteriuria in spinal cord injury patients on condom catheter. J Urol. 1990. 143:979–980.

8. Wybran J, Libin M, Schandene L. Activation of natural killer cells and cytokine production in man by bacterial extracts. Immunopharmacol Immunotoxicol. 1989. 11:17–32.

9. Van Pham T, Kreis B, Corradin-Betz S, Bauer J, Mauël J. Metabolic and functional stimulation of lymphocytes and macrophages by an Escherichia coli extract (OM-89): in vitro studies. J Biol Response Mod. 1990. 9:231–240.
10. Bosch A, Benedi VJ, Pares R, Jofre J. Enhancement of the humoral immune response and resistance to bacterial infection in mice by the oral administration of a bacterial immunomodulator (OM-89). Immunopharmacol Immunotoxicol. 1988. 10:333–343.

11. Tammen H. The German Urinary Tract Infection Study Group. Immunobiotherapy with Uro-Vaxom in recurrent urinary tract infection. Br J Urol. 1990. 65:6–9.

12. Frey C, Obolensky W, Wyss H. Treatment of recurrent urinary tract infections: efficacy of an orally administered biological response modifier. Urol Int. 1986. 41:444–446.

13. Magasi P, Pánovics J, Illés A, Nagy M. Uro-Vaxom and the management of recurrent urinary tract infection in adults: a randomized multicenter double-blind trial. Eur Urol. 1994. 26:137–140.
14. Schulman CC, Corbusier A, Michiels H, Taenzer HJ. Oral immunotherapy of recurrent urinary tract infections: a double-blind placebo-controlled multicenter study. J Urol. 1993. 150:917–921.

15. Tammen H, Frey CH. Treatment of recurrent urinary tract infection with uro-vaxom. Open multicenter study with 521 patients. Urologe B. 1988. 28:294–296.
16. Hauser WE Jr, Remington JS. Effect of antibiotics on the immune response. Am J Med. 1982. 72:711–716.

17. Jacoby GA, Archer GL. New mechanisms of bacterial resistance to antimicrobial agents. N Engl J Med. 1991. 324:601–612.

18. Bottex C, Cristan B, Corazza JL, Mougin B, Fontanges R. Effects of two bacterial extracts OM-89 and Broncho-Vaxom, on IL-I release and metabolic activity of a human macrophage cell line. Int J Immunother. 1988. 4:203–212.
19. Baier W, Sedelmeier EA, Bessler WG. Studies on the immunogenicity of an Escherichia coli extract after oral application in mice. Arzneimittelforschung. 1997. 47:980–985.
20. Nauck M, Matthys H, Emmons LR, Perruchoud A, Reichel H, Pfleger S, Breyer S, Paupe J. The immunomodulators Broncho-Vaxom and Uro-Vaxom stimulate the bacterial killing and oxidative metabolism of polymorphonuclear leukocytes by the activation of phosphatidylinositol turnover. Int J Exp Clin Chemoter. 1991. 4:1–11.
21. Lettgen B. Prevention of recurrent urinary tract infections in female children. Curr Ther Res. 1996. 57:464–475.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download