Abstract
This study examined infectious outcomes in elective colorectal cancer surgery between cefotetan alone or conventional triple antibiotics. From January to December 2007, 461 consecutive primary colorectal cancer patients underwent elective surgery. Group A contained 225 patients who received conventional triple antibiotics (cephalosporin, aminoglycoside and metronidazole) for prophylaxis, and group B contained 236 patients who received cefotetan alone for prophylaxis. Treatment failure was defined as the presence of postoperative infection including surgical-site infection (SSI), anastomotic leakage, and pneumonia or urinary tract infection. The two groups were similar in terms of demographics, American Society of Anesthesiologists (ASA) score, tumour location, stage, surgical approach (conventional open vs. laparoscopy-assisted), and type of operation. The treatment failure rates were 3.1% in Group A and 3.4% in Group B (absolute difference, -0.3%; 95% confidence interval [CI], 0.39 to 3.07, P=0.866), with SSI being the most common reason for failure in both groups (2.7% in Group A and 3.0% in Group B [absolute difference, -0.3%; 95% CI, 0.37 to 3.37, P=0.846]). Cefotetan alone is as effective as triple antibiotics for prophylaxis in primary colorectal cancer patients undergoing elective surgery.
The National Surgical Infection Prevention Project (NSIPP) has recommended prophylactic cefotetan administration to prevent infections in colorectal surgery patients, and cefotetan is currently the most commonly prescribed prophylactic antimicrobial agent in the United States (1). Although traditional triple antibiotic prophylaxis (aminoglycoside, an antianaerobic, and penicillin) is now only used by a small proportion of surgeons in the United States (2), it remains common in other countries (3, 4).
Although many studies have evaluated antimicrobial prophylaxis for preventing infections after colorectal surgery (5-10), none has compared cefotetan alone with traditional triple antibiotic treatment. Our center used a combination of cefazolin, metronidazole and gentamicin as prophylactic antibiotics in colorectal cancer surgery until June 2007. In accordance with the NSIPP recommendations, from July 2007, our institute switched to use cefotetan alone for this purpose. The present study compared infectious outcomes in elective colorectal cancer surgery patients treated with cefotetan alone or conventional triple antibiotics.
From January to December 2007, 648 consecutive patients underwent surgery for primary colorectal cancer at the Center for Colorectal Cancer, National Cancer Center, Korea. The inclusion criteria for the study were: 1) elective surgery, 2) curative intent surgery with standard lymph node dissection, 3) no combined surgery involving other organs, and 4) no evidence of infection at the time of surgery. Of the initial 648 patients, 187 were excluded from the study due to combined surgery with other organs (74), palliative surgery (limited resection or diverting stoma creation only) (64), allergies to penicillin or metronidazole (37), local procedures such as transanal excision or transanal endoscopic microsurgery (10) and emergency surgery (2) for homogenous inclusions. The remaining 461 patients were analyzed for the study. The study was performed in accordance with the guidelines of our Institutional Review Board (NCCNCS-08-131).
Patients were grouped based on their prophylactic antibiotic protocol. Group A=a conventional triple antibiotic combination of cefazolin (Cefamezin, Dong-A Pharm, Seoul, Korea), metronidazole (Trizele, Choongwae Pharma, Seoul, Korea) and gentamicin (Gentamicin, Choongwae Pharma, Seoul, Korea) (this protocol was used until July 2007). Group B=cefotetan (Cefotetan, Kukje Pharm, Seongnam, Korea) alone (this protocol was used from July 2007).
Sodium phosphate (SP) was the most commonly used mechanical bowel preparation (MBP) regimen based on patient tolerance. Polyethylene glycol (PEG) was used in patients >65 yr old or with comorbidity (including compromised renal function or cardiovascular disease). MBP involved ingestion of 2×45 mL SP or 4 L PEG two days before surgery. The methods of patient preparation and antibiotic prophylaxis are summarized in Table 1.
Demographic information including sex, age, body mass index (BMI), medical history such as comorbidities (diabetes mellitus, chronic obstructive pulmonary disease, and ischemic heart disease including angina and myocardial infarction), chemoradiation, oral steroid usage, and information on smoking and alcohol ingestion immediately prior to surgery were collected from all patients. Perioperative information regarding diagnosis, surgical procedures, perioperative (within 2 weeks) transfusions, length of operation and pathology results were also collected.
Data on the following postoperative complications were analyzed: surgery-related infections (i.e., surgical-site infections (SSI) occurring within the first 30 days after the procedure), non-surgery related infection including pneumonia and urinary tract infection, anastomotic leakage, and postoperative ileus. SSI was defined according to a modification of the Centers for Disease Control and Prevention (CDC) (11) and included superficial incisional infection, infection of the deep incision space and organ space infection. Superficial incisional infection was defined as that involving only skin and subcutaneous tissue, and excluded stitch abscesses. Deep incisional infection was defined as that involving deep soft tissues (e.g., fascia and muscle layers). Organ space infection was defined as that involving any part of the anatomy that was opened or manipulated during surgery (e.g., organs or spaces) other than the incision and was diagnosed by abdominal computed tomography (CT) scans. Anastomotic leakage was clinically defined as symptoms and signs of peritonitis caused by anastomosis leakage during the postoperative period. Physical examination (e.g., digital rectal examination), rigid sigmoidoscopy, and/or abdominal CT scans were used to verify the leak.
Postoperative complications including SSI were evaluated by at least two surgeons. Especially, SSI was classified according to the agreement among the surgeons.
Blood samples were obtained from peripheral veins before surgery and on postoperative days 1 and 5. Laboratory parameters such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) levels were analyzed to indicate inflammatory reactions.
Treatment failure was defined as the presence of any postoperative infection including SSI, anastomotic leakage and pneumonia or urinary tract infection.
Statistical analysis involved Pearson's chi-square, Fisher's exact, or Student's t tests, depending on the nature of the data. A two-tailed P value <0.05 was considered to indicate a significant difference.
The demographic and clinical characteristics of patients are shown in Table 2. The two groups showed no significant differences in terms of age, sex, BMI, medical comorbidities, past medical and social history. The two groups were also similar in terms of perioperative transfusions, location of primary tumors, surgery performed, surgery duration, and surgical pathology (Table 3).
Postoperative outcomes are listed in Table 4. There were no postoperative mortalities and anastomotic leakage. The overall morbidity rate was 5.4% (25/461), and the overall treatment failure rate was 3.3% (15/461). The mean length of stay and postoperative morbidity were similar for both groups. The treatment failure rates were also similar, being 3.1% in Group A and 3.4% in Group B (absolute difference, -0.3%; 95% confidence interval [CI], 0.39 to 3.07, P=0.866). The overall SSI rate was 2.8% (13/461), and this was the most common reason for treatment failure in both groups. The SSI rates were similar for the two groups (2.7% in Group A and 3.0% in Group B [absolute difference, -0.3%; 95% CI, 0.37 to 3.37, P=0.846]).
Nine (69%) of the 13 patients with SSI underwent conventional open surgery, while the remaining four underwent laparoscopy-assisted surgery. The SSI rate after laparoscopy-assisted surgery was 1.1% (4/358 patients), and this was lower than after conventional open surgery (8.7%, 9/103) (P<0.05).
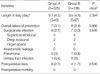
Both groups were similar in terms of pre-operative WBC count, ANC, and CRP levels (Fig. 1). Postoperatively, both groups were similar to each other in terms of WBC count, ANC, and CRP levels at postoperative 1 day, while WBC count and ANC levels were lower in group B than group A at postoperative day 5.
The second-generation cephalosporins have activity against Gram-positive microbes and show improved toxicity against Gram-negative bacteria, compared with that of first-generation cephalosporins. Among the second-generation antibiotics, cefotetan, a cephamycin, has excellent activity against anaerobes (12) and is recommended for prophylaxis in colorectal surgery patients (1). Cefoxitin, like cefotetan, is a cephamycin, a second-generation cephalosporin with an antibacterial spectrum similar to that of cefotetan, and cefoxitin is also recommended for colorectal surgery cases (1). However, cefoxitin has a shorter half-life than cefotetan. A comparative study showed that cefotetan levels remained higher than those of cefoxitin after prolonged surgery (13). Many studies have compared the prophylactic efficacy of cefotetan with that of other antibiotics in elective colorectal surgery (5-10). A recent study (10) showed that ertapenem, a long-acting carbapenem antibiotic with wide coverage against Gram-positive, Gram-negative, and anaerobic microbes, was more effective than cefotetan in the prevention of SSI in patients undergoing elective colorectal surgery, but was more likely to increase the incidence of C. difficile infection. Most studies have reported that the efficacy of cefotetan is comparable to that of other antibacterials, both when cefotetan is compared to other antibiotic combinations (5, 7, 8) and when cefotetan is compared to multiple-dose regimens (6) of other antibacterial compounds. It was thought that combination therapy offered both broad-spectrum antibacterial activity and synergistic effects. However, a meta-analysis (14) found no difference between combination therapy and monotherapy in terms of patient mortality or the rate of resistance development. Furthermore, clinical and bacteriological failures, as well as nephrotoxicity, were more common in patients receiving combination treatments. These findings encouraged us to change our routine antibiotic regimen and to conduct the present study.
This work is a non-randomized comparative study examining the efficacy of cefotetan monotherapy, compared to conventional triple antibiotic prophylaxis, in patients undergoing elective colorectal cancer surgery. However, any bias resulting from non-randomization was minimized by prospective collection of data from two groups of patients who had received different antibiotic regimens. The regimens were applied during different periods, but for the same duration of 6 months, before and after a change of antibiotic regimen.
SSI is the most commonly encountered complication after colorectal surgery. The reported rates of SSI following such surgery range from 4.7% to 26.2% (6, 8-10, 15, 16). This wide variation probably reflects between-study differences in procedures (e.g., stoma-creating procedures, emergency surgery, or multivisceral resection) and clinical settings, and may also reflect differences in infection assessment periods.
Inflammatory bowel disease (IBD), diverticular disease, stoma-creating procedures, multivisceral resection, and emergency surgery can all increase the rate of postoperative infection. In a study of SSI that included all abdominal colorectal operations (for IBD and diverticular disease) and emergency surgery, Blummetti et al. (16) found an SSI rate of 24.5%. Itani et al. (10) excluded patients who required emergency surgery but included those with diverticulitis or IBD. These authors found, in a modified intention-to-treat analysis, that the SSI rate was 17.1% in an ertapenem group and 26.2% in a cefotetan group. In a study of elective colorectal resection patients that included IBD patients (19.9% of all patients) and diverticular disease patients (9.7% of all patients), Smith et al. (15) reported an incisional (superficial or deep) SSI rate of 25.6%. Periti et al. (6) analyzed patients who underwent elective colorectal surgery, including those with IBD or diverticular disease, but excluding patients with severe IBD or diverticular disease complicated by abscess or enterocutaneous fistula. These authors reported SSI rates of 15.5% in cefoxitin-treated patients and 10.1% in cefotetan-treated patients.
The present study excluded patients undergoing emergency surgery, surgery with palliative intent, surgery combined with other organ resection, and local excision for homogenous inclusions. We assessed infections that developed within 30 days of surgery. A 30-day follow-up time is important to obtain reliable data on the incidence of postoperative infections (17, 18). We found the SSI rate to be only 2.8% despite the relatively long assessment period, a figure substantially lower than reported in other studies.
Several factors could account for the relatively low SSI rates observed in the present study. The study excluded patients undergoing "infection-prone" surgical procedures. In addition, there was a high proportion of laparoscopy-assisted operations (78.1%, 360/461 procedures). We found that laparoscopy-assisted surgery patients had lower SSI rates than conventional open surgery patients (1.1% vs. 8.7%). This observation is consistent with the study by Braga et al. (19), who reported that laparoscopic procedures were associated with lower rates of postoperative infections, particularly wound infections, than open procedures, in colorectal surgery cases. This may be because of minimal wound contamination, shorter incisions, and less manipulation of the intestine. The other possible reason is that patients in the present study had a lower average BMI than seen in Western patients. It has been reported that SSI rates were greater in high-BMI than in low-BMI patients (20), and that obesity was associated with SSI in patients undergoing colorectal surgery (10). The mean BMI of our 461 patients was 23.6 kg/m2 (range, 15.2-34.6 kg/m2), and 147 (31.9%) had a BMI ≥25 kg/m2. Only 10 patients (2.2%) had a BMI ≥30 kg/m2. One study performed in the United States (15) reported an incisional SSI rate of 25.6% after elective colorectal resection, which was higher than predicted from the literature. The authors suggested that the high figure may have reflected the large proportion of high BMI patients (54% had BMI ≥25 kg/m2, and 23% had BMI ≥30 kg/m2). The proportion of overweight and obese patients was thus higher in the cited study than in our present work.
To our knowledge, no study has used laboratory data to compare the efficacy of prophylactic antibiotics in colorectal surgery cases. We analyzed WBC count, ANC, and CRP values. Under normal conditions neutrophils comprise about 60% of peripheral WBCs. An alteration in ANC can lead to a change in the total WBC count. The peripheral WBC count, including ANC, is a useful indicator of postoperative infection, because of the important role of neutrophils in combating such infection. Takahashi et al. (21) reported that an increase in neutrophil count several days after surgery is one of the most important signs of bacterial infection. CRP is an important component of the acute-phase response and the CRP response is very consistent (22). Postoperative CRP is an indicator of the degree of trauma or the presence of infection. CRP concentrations rise in the immediate postoperative period (4-12 hr) and reach maximal levels at 24-72 hr (23-25). CRP is more sensitive in indicating postoperative infection than is the WBC count. A recent study (26) evaluating the potential of CRP and WBC data to detect infectious complications after rectal surgery showed that WBC counts did not indicate unfavorable outcomes as early as CRP elevation. In complicated cases, CRP elevation generally persisted after postoperative day 2, whereas WBC counts were within normal ranges in the early postoperative period. We found that our two groups were similar in terms of these laboratory parameters at postoperative day 1, but that both WBC counts and ANCs were lower at postoperative day 5 in group B. However, these two parameters were within normal ranges in both groups. In addition, CRP concentrations, which are more sensitive to infection, decreased to near-normal levels at that time. Overall, the effects of the two antibiotic regimens on the immune response did not differ significantly.
Considering the present finding that cefotetan alone is as effective as triple antibiotics for prophylaxis in elective primary colorectal cancer surgery, we recommend the use of cefotetan alone for such prophylaxis.
Figures and Tables
Fig. 1
Laboratory parameters.
*P<0.05.
Preop, preoperative; POD1, postoperative day 1; POD5, postoperative day 5.

Table 1
Methods of patient preparation and antibiotic prophylaxis

Numbers in parentheses indicate the time of administration.
*Antibiotics were administered per os; †Antibiotics were administered intravenously within 60 min before skin incision; ‡Antibiotics were administered intravenously at 8-hr intervals.
Preop, preoperative day; POD, postoperative day; KM, kanamycin; MTZ, metronidazole; EM, erythromycin; CZL, cefazolin; CTT, cefotetan; GM, gentamicin.
References
1. Bratzler DW, Houck PM, Richards C, Steele L, Dellinger EP, Fry DE, Wright C, Ma A, Carr K, Red L. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005. 140:174–182.
2. Zmora O, Wexner SD, Hajjar L, Park T, Efron JE, Nogueras JJ, Weiss EG. Trends in preparation for colorectal surgery: survey of the members of the American Society of Colon and Rectal Surgeons. Am Surg. 2003. 69:150–154.
3. Shpitz B, Reissman P, Rabau M, Ziv Y. Perioperative management of patients undergoing elective colorectal surgery in Israel: a national survey. Surg Infect (Larchmt). 2005. 6:305–312.

4. Aoun E, El Hachem S, Abdul-Baki H, Ayyach B, Khalifeh M, Chaar H, Kanafani ZA, Kanj SS, Sharara AI. The use and abuse of antibiotics in elective colorectal surgery: the saga continues. Int J Surg. 2005. 3:69–74.

5. Bellantone R, Pacelli F, Sofo L, Doglietto GB, Bossola M, Ratto C, Crucitti F. Systemic perioperative prophylaxis in elective oncological colorectal surgery: cefotetan versus clindamicin plus aztreonam. Drugs Exp Clin Res. 1988. 14:763–766.
6. Periti P, Mazzei T, Tonelli F. Single-dose cefotetan vs. multiple-dose cefoxitin--antimicrobial prophylaxis in colorectal surgery. Results of a prospective, multicenter, randomized study. Dis Colon Rectum. 1989. 32:121–127.
7. Skipper D, Karran SJ. A randomized prospective study to compare cefotetan with cefuroxime plus metronidazole as prophylaxis in elective colorectal surgery. J Hosp Infect. 1992. 21:73–77.

8. Arnaud JP, Bellissant E, Boissel P, Carlet J, Chastang C, Lafaix C, Rio Y, Berganeschi R. The PRODIGE Group. Single-dose amoxycillin-clavulanic acid vs. cefotetan for prophylaxis in elective colorectal surgery: a multicentre, prospective, randomized study. J Hosp Infect. 1992. 22:Suppl A. 23–32.
9. Song F, Glenny AM. Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomized controlled trials. Br J Surg. 1998. 85:1232–1241.

10. Itani KM, Wilson SE, Awad SS, Jensen EH, Finn TS, Abramson MA. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med. 2006. 355:2640–2651.

11. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992. 20:271–274.

12. Kendler JS, Hartman BJ. Cohen J, Powderly W, editors. Beta-lactam antibiotics. Infectious disease. 2003. 2nd ed. New York: Mosby;1773.
13. Quintiliani R, Nightingale CH, Stevens RC, Outman WR, Deckers PJ, Martens MG. Comparative pharmacokinetics of cefotetan and cefoxitin in patients undergoing hysterectomies and colorectal operations. Am J Surg. 1988. 155:67–70.

14. Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004. 328:668.
15. Smith RL, Bohl JK, McElearney ST, Friel CM, Barclay MM, Sawyer RG, Foley EF. Wound infection after elective colorectal resection. Ann Surg. 2004. 239:599–605.

16. Blumetti J, Luu M, Sarosi G, Hartless K, McFarlin J, Parker B, Dineen S, Huerta S, Asolati M, Varela E, Anthony T. Surgical site infections after colorectal surgery: do risk factors vary depending on the type of infection considered? Surgery. 2007. 142:704–711.

17. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001. 358:876–880.

18. Keeling NJ, Morgan MW. Inpatient and post-discharge wound infections in general surgery. Ann R Coll Surg Engl. 1995. 77:245–247.
19. Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002. 236:759–766.
21. Takahashi J, Ebara S, Kamimura M, Kinoshita T, Itoh H, Yuzawa Y, Sheena Y, Takaoka K. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine. 2001. 26:1698–1704.

22. Crockson RA, Payne CJ, Ratcliff AP, Soothill JF. Time sequence of acute phase reactive proteins following surgical trauma. Clin Chim Acta. 1966. 14:435–441.

23. Aronsen KF, Ekelund G, Kindmark CO, Laurell CB. Sequential changes of plasma proteins after surgical trauma. Scand J Clin Lab Invest Suppl. 1972. 124:127–136.

24. Pullicino EA, Carli F, Poole S, Rafferty B, Malik ST, Elia M. The relationship between the circulating concentrations of interleukin 6 (IL-6), tumor necrosis factor (TNF) and the acute phase response to elective surgery and accidental injury. Lymphokine Res. 1990. 9:231–238.
25. Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, Gotoh M, Kishimoto T, Mori T. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992. 111:201–209.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download