Abstract
Eradication regimens for Helicobacter pylori infection have some side effects, compliance problems, relapses, and antibiotic resistance. Therefore, alternative anti-H. pylori or supportive antimicrobial agents with fewer disadvantages are necessary for the treatment of H. pylori. We investigated the pH-(5.0, 6.0, 7.0, 8.0, 9.0, and 10.0) and concentration (0.032, 0.064, 0.128, 0.256, 0.514, and 1.024 mg/mL)-dependent antibacterial activity of crude urushiol extract from the sap of the Korean lacquer tree (Rhus vernicifera Stokes) against 3 strains (NCTC11637, 69, and 219) of H. pylori by the agar dilution method. In addition, the serial (before incubation, 3, 6, and 10 min after incubation) morphological effects of urushiol on H. pylori were examined by electron microscopy. All strains survived only within pH 6.0-9.0. The minimal inhibitory concentrations of the extract against strains ranged from 0.064 mg/mL to 0.256 mg/mL. Urushiol caused mainly separation of the membrane, vacuolization, and lysis of H. pylori. Interestingly, these changes were observed within 10 min following incubation with the 1×minimal inhibitory concentrations of urushiol. The results of this work suggest that urushiol has potential as a rapid therapeutic against H. pylori infection by disrupting the bacterial cell membrane.
Helicobacter pylori infection is associated with chronic gastritis, gastric and duodenal ulcers, and gastric cancer in humans (1, 2). Eradication of H. pylori results in ulcer healing, reduction of recurrence, and complete cure of lymphomas. Several drug treatment regimens have been developed to eradicate H. pylori with a cure rate of up to 90% (3). Antibiotics, proton pump inhibitors, H2 blockers, and bismuth salts are the typical modalities of treatment (4). However, these regimens have some side effects, compliance problems, relapses, and antibiotic resistance. Therefore, alternative anti-H. pylori or supportive antimicrobial agents with fewer disadvantages are necessary for the treatment of H. pylori.
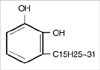
Urushiol is an oil found in plants of the Family Anacardiaceae and a major organic component of the sap of the lacquer tree (Rhus vernicifera Stokes). It causes an allergic skin rash, inflammation, and irritation on contact, known as urushiol-induced contact dermatitis. Urushiol is a yellow liquid with a boiling point of 200-210℃. It is miscible partially in alcohol and ether, but nearly immiscible in water. Chemically, urushiol is a mixture of several closely related organic compounds. Each consists of a catechol substituted with an alkyl chain that has 15 or 17 carbon atoms (Fig. 1). The alkyl group may be saturated or unsaturated; urushiol oil is a mixture of saturated and unsaturated molecules (5).
Traditionally, despite an allergic reaction to urushiol and lack of rigorous documentation, the lacquer tree was used as a folk remedy for the relief of abdominal discomfort or dyspepsia when administered mixed with boiled chicken in Korea. Moreover, some reports have demonstrated that urushiol has both anti-microbial and anti-oxidative activities (6, 7). In this study, we evaluated the pH- and concentration-dependent antibacterial activity of urushiol from the sap of the lacquer tree against 3 strains of H. pylori.
The sap (40 mL) of lacquer tree was diluted to a volume of 1 L by the addition of distilled water and extracted with 1 L of n-hexane twice. The hexane extract was concentrated under reduced pressure to yield a brownish oil (26.9 g). The oil was then purified by silica gel column chromatography (Merck 7734) and eluted with 20% acetone/hexane. It was further purified by the same method (Merck 9385), followed by octadecyl silica gel column chromatography (YMC GEL ODS-A) using a gradient of methanol in water to give urushiols. The final concentration of extracted urushiol was 10 mg/mL.
Three strains of H. pylori (H. pylori NCTC11637, H. pylori 69 [containing plasmid pBHP69KH: kanamycin resistant strain] and H. pylori 219 [ggt isogenic mutant: chloramphenicol resistant and urease-negative strain]) were obtained from the H. pylori Korean Type Culture Collection (HpKTCC, Department of Microbiology, Gyeongsang University College of Medicine, Jinju, Korea).
H. pylori was isolated from antral mucosal biopsies of patients with chronic gastritis and peptic ulcers. The H. pylori strain was identified by colony morphology, Gram staining, motility tests, and biochemical assays such as the urease test, the catalase test, or the oxidase test. H. pylor was cultured in an atmosphere 10% CO2, 5% O2, and 100% humidity at 37℃. For transport, stab cultures were grown in Wang's media (7.0 g brucella broth, 1.25 g agar [Difco] and 250 mL distilled water) and then frozen in cryomedia in the deep freezer (-70℃). For our study, the frozen strains were processed by thawing, revitalizing, and lyophilizing. Every 72 hr, we subcultured H. pylori strains on Muller-Hinton agar with aged (>2 weeks old) 5% sheep blood and incubated in a CAMP pouch (7% CO2) in a 37℃/5% CO2 incubator. After subculturing twice, we checked the minimal inhibitory concentration of urushiol at different pH conditions and evaluated the morphological effects of urushiol on H. pylori.
The agar dilution method was used. Various concentrations of urushiol liquid (0.032, 0.064, 0.128, 0.256, 0.514, and 1.024 mg/mL) were mixed with Mueller-Hinton agar with aged (>2 weeks old) 5% sheep blood. A saline suspension equivalent to a 2.0 McFarland standard (containing 1×107 to a 1×108 CFU/mL) was prepared from a 72-hr-old subculture from a blood agar plate. The inoculum (1 to 3 L per spot) was replicated directly onto the urushiol-containing agar dilution plates. The plates were kept in a micro-aerobic atmosphere produced by the CAMP pouch (7% CO2) in a 37/5% CO2 incubator for 3 days. After 3 days incubation, we checked the minimal inhibitory concentration. We then evaluated the minimal inhibitory concentration values of urushiol following this same procedure under different pH (5.0, 6.0, 7.0, 8.0, 9.0, and 10.0) conditions.
For the ultra-structural analysis, H. pylori was incubated in urushiol (0.256 mg/mL) mixed agar. Initial (before incubation) and serial fixations (3, 6, and 10 min after incubation) were done in phosphate-buffered saline containing 2.5% glutaraldehyde.
For scanning electron microscopy observations, the skin specimens were fixed in 2.5% glutaraldehyde for 2 hr at 4℃, washed in 0.1 M phosphate-buffered saline (pH 7.4), postfixed in 1% osmium tetroxide in the same buffer for 90 min at 4℃, dehydrated in a series of ethyl alcohol, exchanged through isoamylacetate, and critical-point dried. The specimens were coated with gold-palladium and then examined under a scanning electron microscope (Tabletop microscope, TM-1000, Hitachi high technologies, Tokyo, Japan) at 30 kV.
For transmission electron microscopy observations, the specimens were fixed in 2.5% glutaraldehyde for 2 hr at 4℃, washed in 0.1 M phosphate-buffered saline (pH 7.4), and then postfixed in 1% osmium tetroxide in the same buffer for 90 min. The specimens were then dehydrated through a graded series of ethanol, exchanged through propylene oxide, and embedded in a mixture of Epon. Subsequently, ultrathin sections were obtained by an ultramicrotome (Richert-Jung, RMC Inc., Fresno, CA, USA) with a diamond knife. Ultrathin sections were double stained with uranyl acetate and lead citrate and examined under a transmission electron microscope (JEOL-1200EX II, JEOL LTD., Tokyo, Japan) at 80 kV.
The minimal inhibitory concentration (mg/mL) of the urushiol against 3 H. pylori strains ranged from 0.064 to 0.256. The minimal inhibitory concentration values of kanamycin-resistant H. pylori (H. pylori 69) and other strains (H. pylori NCTC11637 and H. pylori 219) were nearly equal. All strains survived only within pH 6.0-9.0. In media of pH 5 or 10, H. pylori did not survive after 3 days incubation, irrespective of the concentration of urushiol (Table 1).
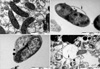
In the results of initial (before incubation) and serial fixations (3, 6, and 10 min after incubation), separation of the membrane, vacuolization, and lysis of H. pylori were the serial morphological changes of H. pylori under 1×minimal inhibitory concentration of urushiol. After 3 min exposure, separation of the cell wall and vacuole and bleb formation were observed. Leakage of some cellular material from the cytoplasmic membrane was observed. At 6 min after exposure, significant separation with secretory granule loss and lysis of the cytoplasmic membrane were observed. After 10 min exposure, the bacteria are almost complete lysed (Figs. 2, 3).
Because antibiotic resistance is a major factor affecting the outcome of treatment, many non-antibiotic therapies including phytomedicines, probiotics, and antioxidants have been investigated as potential alternatives for the treatment of H. pylori infections (8-12). The minimal inhibitory concentrations of these alternatives against H. pylori have been reported as ranged from 0.075 to 12.5 mg/mL. In our study, the minimal inhibitory concentration of the urushiol against 3 H. pylori strains ranged from 0.064 to 0.256 mg/mL. When compared with previous reports of minimal inhibitory concentration of methanol, myrobalan, and plants extracts (9, 13, 14), The result of the present study suggest that urushiol could be a new candidate in the treatment of H. pylori infections.
When survival of H. pylori was examined under different pH conditions, all strains survived in pH range 6.0-9.0. Because antibiotics have different minimal inhibitory concentrations depending upon pH (15, 16), H. pylori eradication regimens using antibiotics need to include proton pump inhibitors. However, the minimal inhibitory concentration values of urushiol revealed minimal differences under different pH conditions. As a result, urushiol may not be influenced by pH and could be a supplementary agent for an anti-H. pylori regimen without proton pump inhibitor.
We evaluated the effect of urushiol on H. pylori with kanamycin resistance. The minimal inhibitory concentration of urushiol in kanamycin-resistant H. pylori and other two strains were nearly equal. In addition, the minimal inhibitory concentration of urushiol in H. pylori 219 (urease-negative strain) was not different from that of urease positive H. pylori. Though H. pylori survive in the stomach due to its urease activity (17), the presence of urease activity is not related in antibacterial effect of urushiol on H. pylori in this study. These findings confirm that urushiol has a different mechanism on H. pylori eradication.
At 6 min following incubation with 1×minimal inhibitory concentration of urushiol, most of the H. pylori bacteria have changed to a round shape and are lysed. Within 10 min after exposure, separation of the membrane, vacuolization, and lysis of H. pylori were sequentially observed. Peroxynitrite was reported to cause morphological changes of H. pylori within 15 min (10), while some antibiotics were reported to act on the H. pylori within 10 hr (8). Based on these reports and the present study results, relative activity of urushiol against H. pylori seems to be quick. The results of our work suggest that urushiol has the potential to be used as a rapid acting therapeutic against H. pylori infection. In addition, we could expect that urushiol acts by disrupting the H. pylori cell membrane. For the further evaluation of action mechanism, studies using gold tagging urushiol or fluorescent microscope are warranted.
Based on results from the present study, we would suspect that urushiol is effective in H. pylori eradication and reduction of H. pylori gastritis or ulcer. However, a key problem of urushiol compared with other new treatment modalities (18-21) is a hypersensitive or allergic reaction. Some studies reported that allergic adverse effect of urushiol could be prevented by modification or polymerization of chemical structure (22, 23). Therefore, further preclinical or clinical studies using chemically modified non-allergic urushiol are needed. In this point, this in-vitro study is a basic proposal that urushiol maybe an alternative therapeutic agent for the H. pylori eradication in the future.
In conclusion, urushiol has the potential to act rapidly against H. pylori infection by disrupting the H. pylori cell membrane.
Figures and Tables
Fig. 2
Serial scanning electron micrographs of H. pylori exposed to 1×minimal inhibitory concentration of urushiol. (A) Without urushiol, most of the H. pylori bacteria are in spiral forms. (B) After 3 min exposure to urushiol, some of the H. pylori bacteria are still in the spiral form. (C) At 6 min after exposure, most of the H. pylori bacteria have changed to a round shape and are lysed. (D) After 10 min exposure, almost complete lysis of H. pylori bacteria is observed.

Fig. 3
Serial transmission electron micrographs of H. pylori exposed to 1×minimal inhibitory concentration of urushiol. (A) In control without urushiol exposure, H. pylori bacteria are in normal bacillary form. (B) After 3 min exposure, separation of the cell wall and vacuole and bleb formation are observed (arrow). Leakage of some cellular material from the cytoplasmic membrane is observed (arrow head). (C) At 6 min after exposure, significant separation with secretory granule loss and lysis of the cytoplasmic membrane are observed (arrow). (D) After 10 min exposure, the bacteria are almost complete lysed (arrow).
References
1. Ferreira AC, Isomoto H, Moriyama M, Fujioka T, Machado JC, Yamaoka Y. Helicobacter and gastric malignancies. Helicobacter. 2008. 13:Suppl 1. 28–34.
2. Kandulski A, Selgrad M, Malfertheiner P. Helicobacter pylori infection: a clinical overview. Dig Liver Dis. 2008. 40:619–626.

3. Marzio L, Cellini L, Angelucci D. Triple therapy for 7 days vs. triple therapy for 7 days plus omeprazole for 21 days in treatment of active duodenal ulcer with Helicobacter pylori infection. A double blind placebo controlled trial. Dig Liver Dis. 2003. 35:20–23.
4. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007. 56:772–781.

5. Elsohly MA, Adawadkar PD, Benigni DA, Watson ES, Little TL Jr. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986. 29:606–611.
6. Kim MJ, Choi YH, Kim WG, Kwak SS. Antioxidative activity of urushiol derivatives from the sap of lacquer tree. Korean J Plant Resour. 1997. 10:227–230.
7. Kim MJ, Kim CJ, Kwak SS. Antifungal activity of urushiol component in the sap of Korean lacquer tree (Rhus vernicifera Stokes). Korean J Plant Resour. 1997. 10:231–234.
8. Horii T, Mase K, Suzuki Y, Kimura T, Ohta M, Maekawa M, Kanno T, Kobayashi M. Antibacterial activities of beta-lactamase inhibitors associated with morphological changes of cell wall in Helicobacter pylori. Helicobacter. 2002. 7:39–45.

9. Adeniyi BA, Anyiam FM. In vitro anti-Helicobacter pylori potential of methanol extract of Allium ascalonicum Linn. (Liliaceae) leaf: susceptibility and effect on urease activity. Phytother Res. 2004. 18:358–361.

10. Tecder-Unal M, Can F, Demirbilek M, Karabay G, Tufan H, Arslan H. The bactericidal and morphological effects of peroxynitrite on Helicobacter pylori. Helicobacter. 2008. 13:42–48.
11. Stamatis G, Kyriazopoulos P, Golegou S, Basayiannis A, Skaltsas S, Skaltsa H. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J Ethnopharmacol. 2003. 88:175–179.

12. Kamiji MM, de Oliveira RB. Non-antibiotic therapies for Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2005. 17:973–981.

13. Malekzadeh F, Ehsanifar H, Shahamat M, Levin M, Colwell RR. Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int J Antimicrob Agents. 2001. 18:85–88.

14. Nostro A, Cellini L, Di Bartolomeo S, Di Campli E, Grande R, Cannatelli MA, Marzio L, Alonzo V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother Res. 2005. 19:198–202.
15. Lascols C, Bryskier A, Soussy CJ, Tankovic J. Effect of pH on the susceptibility of Helicobacter pylori to the ketolide telithromycin (HMR 3647) and clarithromycin. J Antimicrob Chemother. 2001. 48:738–740.
16. Hassan IJ, Stark RM, Greenman J, Millar MR. Activities of beta-lactams and macrolides against Helicobacter pylori. Antimicrob Agents Chemother. 1999. 43:1387–1392.
17. McGowan CC, Cover TL, Blaser MJ. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996. 110:926–938.

18. Park MJ, Kim JS, Yim JY, Jung HC, Song IS, Yu ES, Lee JJ, Huh CS, Baek YJ. The suppressive effect of a fermented milk containing lactobacilli on Helicobacter pylori in human gastric mucosa. Korean J Gastroenterol. 2001. 38:233–240.
19. Wang YC, Huang TL. Anti-Helicobacter pylori activity of Plumbago zeylanica L. FEMS Immunol Med Microbiol. 2005. 43:407–412.
20. Millar MR, Pike J. Bactericidal activity of antimicrobial agents against slowly growing Helicobacter pylori. Antimicrob Agents Chemother. 1992. 36:185–187.

21. Blacky A, Makristathis A, Apfalter P, Willinger B, Rotter ML, Hirschl AM. In vitro activity of fosfomycin alone and in combination with amoxicillin, clarithromycin and metronidazole against Helicobacter pylori compared with combined clarithromycin and metronidazole. Eur J Clin Microbiol Infect Dis. 2005. 24:276–279.

22. Xia Z, Miyakoshi T, Yoshida T. Lipoxygenase-catalyzed polymerization of phenolic lipids suggests a new mechanism for allergic contact dermatitis induced by urushiol and its analogs. Biochem Biophys Res Commun. 2004. 315:704–709.

23. Harigaya S, Honda T, Rong L, Miyakoshi T, Chen CL. Enzymatic dehydrogenative polymerization of urushiols in fresh exudates from the lacquer tree, Rhus vernicifera DC. J Agric Food Chem. 2007. 55:2201–2208.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download