Abstract
Pulmonary toxicity is one of the most serious adverse effects associated with a quick course of vincristine, bleomycin, and cisplatin neoadjuvant chemotherapy (NAC-VBP). The aim of this study was to evaluate pulmonary toxicity related to a quick course NAC-VBP. A total of consecutive 61 patients, who underwent at most 3 cycles of NAC-VBP every 10 days in the International Federation of Gynecology and Obstetrics (FIGO) stage IB-IIB cervical cancer from 1995 to 2007, were retrospectively analyzed. Of the 61 study subjects, 7 (11.5%) were identified to have pulmonary toxicity and 2 (3.3%) died of pulmonary fibrosis progression despite aggressive treatment and the use of a multidisciplinary approach. No factor predisposing pulmonary toxicity was identified. Initial symptoms were non-specific, but bronchiolitis obliterans organizing pneumonia and interstitial pneumonitis were characteristic findings by high-resolution computed tomography of the chest. The benefit of steroid therapy was uncertain and was associated with steroid-induced diabetes mellitus requiring insulin therapy in two patients. Fatal pulmonary toxicity is a major concern of a quick course NAC-VBP. In conclusion, these patients require special monitoring for bleomycin-induced pulmonary toxicity.
Uterine cervical cancer is the most common malignancy of the female genital tract, and in 2002 accounted for 9.1% of total malignancies in Korean women (1). Neoadjuvant chemotherapy (NAC) based on cisplatin has been widely used as a therapeutic strategy to improve survival in locally advanced cervical cancer (2-4). Of the several available NAC regimens, a quick (at most three cycles) course of vincristine, bleomycin, and cisplatin is to be preferred due to short duration. NAC administered every 10 days has been tried and found to produce favorable results (5, 6). Furthermore, bleomycin-induced pulmonary toxicity, one of the most fatal adverse effects, has been reported to be unusual after a quick course of NAC-VBP (5-7). However, we have experienced several fatal cases of pulmonary toxicity, at levels higher than those reported in the literature (5-10), associated with a quick course of NAC-VBP in patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB-IIB cervical cancer.
In the present study, we analyzed our experiences of radiologically and/or histologically confirmed pulmonary toxicity following a quick course of NAC-VBP in cervical cancer.
After obtaining the approval of the Institutional Review Board of Kyung Hee University (Seoul), 61 patients who underwent a quick course of NAC-VBP in FIGO stage IB-IIB cervical cancer from 1995 to 2007 were retrospectively identified in the tumor registry databases at the Department of Obstetrics and Gynecology. The NAC schedule consisted of cisplatin 50 mg/m2 intravenous infusion on day 1, vincristine 1 mg/m2 intravenous bolus on day 1, and bleomycin 25 mg/m2/day continuous intravenous infusion on days 1 to 3. Cycles were repeated every 10 days for a maximum of three cycles. Patients were hospitalized for each chemotherapy period. Prior to administering chemotherapy, complete medical assessments, including chest radiography, creatinine clearance, renal function, and pulmonary function testing (PFT), were performed. Toxicities were measured using WHO criteria (11). Intensive work ups, such as, high-resolution computed tomography (HR-CT) with or without bronchoscopic biopsy, were performed in all cases with pulmonary symptoms or suspicious findings by chest radiography during or after NAC-VBP. Histopathologic slides were reviewed by pathologists at our institute with considerable experience of gynecologic malignancies and pulmonary disease.
A total of 61 patients underwent a quick course of NAC-VBP. No patient had a history of thoracic radiation therapy. Seven (11.5%) of the 61 patients were identified to have pulmonary toxicity and 2 (3.3%) succumbed due to progression of pulmonary fibrosis despite aggressive treatment and the adoption of a multidisciplinary approach.
A comparison of the demographic factors of patients with and without pulmonary toxicity identified no significant differences in terms of age, parity, histologic type, or total bleomycin dose (Table 1).
The histories of 7 patients with pulmonary toxicity are briefly summarized in Table 2. All 7 patients had normal renal and pulmonary functions before NAC-VBP. Pulmonary toxicity was first suspected based on clinical symptoms or routine chest-radiographic and/or pulmonary function test findings, and was subsequently confirmed by HR-CT with or without a bronchoscopic biopsy. Three patients were completely asymptomatic in terms of respiratory symptoms but had suspicious chest radiographic findings. Of the 4 symptomatic patients, 1 suffered from a fever and sputum production, and 3 complained of pulmonary symptoms such as dyspnea and/or cough. Two of the 3 asymptomatic patients were closely observed and spontaneously improved, but 1 patient deteriorated gradually and eventually found it hard to breathe. She was treated with medication containing corticosteroid (prednisolone 1 mg/kg/day). However, her symptom persisted, and thus, corticosteroid was continued at a reduced dose (prednisolone 0.5 mg/kg/day). One patient with fever and sputum production improved on conservative management. In the 3 patients who complained of dyspnea and pulmonary symptoms, 1 improved on steroid therapy (methylprednisolone 40 mg/day, followed by maintenance therapy methylprednisolone 10 mg/day). However, the other 2 patients deteriorated and pulmonary fibrosis progression despite steroid pulse therapy (methylprednisolone 500 mg/day for 3 days) and developed acute respiratory distress syndrome, which led to respiratory arrest and death in both within 1 month of diagnosing pulmonary toxicity. In these two patients, bronchiolitis obliterans organizing pneumonia (BOOP) or interstitial pneumonitis were characteristic chest HR-CT findings. These findings of BOOP were characterized by multifocal, patchy distributions of ground glass attenuation and consolidation, predominantly in the subpleural region, with partial bronchovascular bundle thickening and interlobular septal thickening by HR-CT (Fig. 1). The presence of interstitial pneumonitis was demonstrated by diffuse extensive homogenous ground glass attenuation, which was more prominent in the subpleural region, intermixed with interlobular septal thickenings, bronchiectasis, and honeycomb cysts. These findings suggested diffuse alveolar damage or acute phases interstitial lung disease, such as, nonspecific interstitial pneumonia (Fig. 2). Alveolar damage was confirmed by bronchoscopic biopsies (Fig. 3). Furthermore, as a result of long term steroid use, steroid-induced diabetes mellitus requiring insulin therapy developed in two patients (cases 2 and 5).
It remains unclear whether NAC improves survival outcomes as compared with primary radical surgery or concurrent chemoradiation therapy in cervical cancer (7, 9, 12-14). Nevertheless, many gynecologic oncologists perform NAC to reduce tumor volume and consequently to improve survival, based on clinician's preference and the medical resources available, and thus, the usefulness of NAC is probably beyond the scope of this study. Rather, we focus on pulmonary toxicity, one of the most fatal adverse effects following a quick course of NAC-VBP.
Pulmonary toxicity exacerbation by combination chemotherapy has already been reported upon (15), and of several chemotherapeutic agents responsible, bleomycin has been identified as the most causative agent (16). Pulmonary toxicity is the most fatal adverse effect of bleomycin, and may occur in up to 46% of patients (17, 18), with a mortality rate, among those treated, of approximately 3% (19, 20). However, pulmonary toxicity is rare after a quick NAC-VBP course, which is one of the most popular NAC regimens used in cervical cancer (6-10, 21, 22). Table 3 itemizes publications on pulmonary toxicity after a quick course of NAC-VBP in cervical cancer. The majority of series reported pulmonary toxicity in only 0-2% of the population studied, and that it occurred more frequently in elderly patients (5, 21). However, another study reported that a quick course of NAC-VBP in cervical cancer was associated with significant pulmonary toxicity in 13.3% of cases (22), which is similar to the present study. These findings stress that the possibility of pulmonary toxicity should be considered in patients receiving a quick course of NAC-VBP.
Bleomycin is available in two forms, i.e., as a sulfate and chlorohydrate. In the present study, we used bleomycin chlorohydrate which causes less pulmonary toxicity (5, 21). However, the mechanisms of bleomycin-induced pulmonary toxicity are not entirely understood, though several factors, including old age (>70 yr), a high cumulative dose, impaired renal function, administration route, smoking, granulocyte colony stimulating factor (G-CSF) use, and multiple chemotherapeutics agents (mainly cisplatin) may increase its risk (17). In the present study, all patients with pulmonary toxicity were nonsmokers and were confirmed to have normal renal function by creatinine clearance before each chemotherapy cycle. In addition, we found no evidence that age and cumulative bleomycin dose were significantly different for patients with/without pulmonary toxicity, and thus, we failed to identify any factor predisposing pulmonary toxicity.
Bleomycin-induced pulmonary toxicity may be reversible in cases with minimal alveolar change, but in its advanced state may progress to severe fibrosis and death (23). Moreover, although high doses of corticosteroids have been widely administered in cases of bleomycin-induced pulmonary toxicity, no standard therapy has been agreed. In addition, the efficacies of corticosteroids are unclear and the adverse effects of long term corticosteroid therapy, such as, steroidinduced diabetes mellitus may be grave (18).
We conclude fatal pulmonary toxicity is a major concern of quick NAC-VBP regimens, and advise that special attention be taken to monitor for bleomycin-induced pulmonary toxicity.
Figures and Tables
 | Fig. 1Case 6, Chest HR-CT of 1 week after surgery revealing bronchiolitis obliterans organizing pneumonia. |
 | Fig. 2Case 7, Chest HR-CT of 2 month after surgery revealing diffuse alveolar damage with underlying interstitial lung disease. |
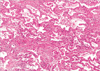 | Fig. 3Case 2, Photomicrograph revealing interstitial widening with chronic inflammatory cell infiltration and foci of intraalveolar fibroblastic plugs (H&E stain, ×100), Lung biopsy. |
References
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004. 36:103–114.

2. Kim DS, Moon H, Hwang YY, Cho SH. Preoperative adjuvant chemotherapy in the treatment of cervical cancer stage Ib, IIa, and IIb with bulky tumor. Gynecol Oncol. 1988. 29:321–332.

3. Weiner SA, Aristizabal S, Alberts DS, Surwit EA, Deatherage-Deuser K. A phase II trial of mitomycin, vincristine, bleomycin, and cisplatin (MOBP) as neoadjuvant therapy in high-risk cervical carcinoma. Gynecol Oncol. 1988. 30:1–6.

4. Panici PB, Scambia G, Baiocchi G, Greggi S, Ragusa G, Gallo A, Conte M, Battaglia F, Laurelli G, Rabitti C. Neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer. Prognostic factors for response and survival. Cancer. 1991. 67:372–379.

5. Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, Snaidas L, Vighi S, Gomez Rueda N, di Paola G. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecol Oncol. 1997. 67:61–69.

6. Lai CH, Hsueh S, Chang TC, Tseng CJ, Huang KG, Chou HH, Chen SM, Chang MF, Shum HC. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 1997. 64:456–462.

7. Chang TC, Lai CH, Hong JH, Hsueh S, Huang KG, Chou HH, Tseng CJ, Tsai CS, Chang JT, Lin CT, Chang HH, Chao PJ, Ng KK, Tang SG, Soong YK. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J Clin Oncol. 2000. 18:1740–1747.

8. Porzio G, Ficorella C, Toro G, Paris I, Ricevuto E, Marchetti P. Short-term weekly neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer. Tumori. 2001. 87:25–26.

9. Huang HJ, Chang TC, Hong JH, Tseng CJ, Chou HH, Huang KG, Lai CH. Prognostic value of age and histologic type in neoadjuvant chemotherapy plus radical surgery for bulky (>/=4 cm) stage IB and IIA cervical carcinoma. Int J Gynecol Cancer. 2003. 13:204–211.
10. Singh KC, Agarwal A, Agarwal S, Rajaram S, Goel N, Agarwal N. 'Quick course' neoadjuvant chemotherapy with cisplatin, bleomycin and vincristine in advanced cervical cancer. Gynecol Obstet Invest. 2004. 58:109–113.

11. World Health Organization. WHO handbook for reporting results of cancer treatment: WHO offset publication. 1979. Geneva, Switzerland:
12. Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F, Zola P, Mangioni C, Landoni F. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. J Clin Oncol. 2002. 20:179–188.

13. Behtash N, Mehrdad N. Cervical cancer: screening and prevention. Asian Pac J Cancer Prev. 2006. 7:683–686.
14. Eddy GL, Bundy BN, Creasman WT, Spirtos NM, Mannel RS, Hannigan E, O'Connor D. Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecol Oncol. 2007. 106:362–369.

15. Kreisman H, Wolkove N. Pulmonary toxicity of antineoplastic therapy. Semin Oncol. 1992. 19:508–520.
17. Azambuja E, Fleck JF, Batista RG, Menna Barreto SS. Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther. 2005. 18:363–366.

19. Simpson AB, Paul J, Graham J, Kaye SB. Fatal bleomycin pulmonary toxicity in the west of Scotland 1991-95: a review of patients with germ cell tumours. Br J Cancer. 1998. 78:1061–1066.

20. Levi JA, Raghavan D, Harvey V, Thompson D, Sandeman T, Gill G, Stuart-Harris R, Snyder R, Byrne M, Kerestes Z. Australasian Germ Cell Trial Group. The importance of bleomycin in combination chemotherapy for good-prognosis germ cell carcinoma. J Clin Oncol. 1993. 11:1300–1305.
21. Sardi JE. Phase II trial with neoadjuvant chemotherapy. Gynecol Oncol. 1996. 62:321–322.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download