Abstract
Cornelia de Lange Syndrome (CdLS) is a multiple congenital anomaly characterized by distinctive facial features, upper limb malformations, growth and cognitive retardation. The diagnosis of the syndrome is based on the distinctive clinical features. The etiology is still not clear. Mutations in the sister chromatid cohesion factor genes NIPBL, SMC1A (also called SMC1L1) and SMC3 have been suggested as probable cause of this syndrome. We experienced a case of newborn with CdLS showing bushy eyebrows and synophrys, long curly eyelashes, long philtrum, downturned angles of the mouth and thin upper lips, cleft palate, micrognathia, excessive body hair, micromelia of both hands, flexion contracture of elbows and hypertonicity. We detected a NIPBL gene mutation in a present neonate with CdLS, the first report in Korea.
Cornelia de Lange Syndrome (CdLS) is a multiple congenital anomaly characterized by distinctive facial features, upper limb malformations, growth and cognitive retardation (1, 2). The syndrome has unknown genetic and molecular pathogenesis. Diagnosis is based on the distinctive clinical features and lack of a definitive laboratory marker. Most cases are sporadic, although autosomal dominant inheritance mutations in Nipped-B homolog (NIPBL), which is located at 5p13, have been suggested (3, 4). Since CdLS was first described in Korea in 1967, several cases have been reported (5). The majority of these previous reports concerned the accompanying anomaly associated with CdLS. These included a case of CdLS with imperforate anus and a case of CdLS with entropion, which was reported by a pediatrician and ophthalmologist respectively (6, 7). However, there is no report regarding the genetic analysis of CdLS in the Korean population. The present report is the first description of a NIPBL gene mutation in a neonate with CdLS in Korea.
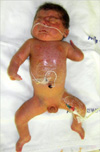
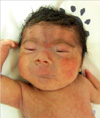
A 1,840 g male was delivered via normal vaginal delivery in our hospital at 32 weeks gestational age at May 2009. His mother was 40-yr-old, and had gestational diabetes mellitus and polyhydramnios. Apgar scores were 2 and 4 at 1 and 5 min, respectively. The patient presented with cyanosis and respiratory difficulty requiring continuous positive airway pressure. No evidence of respiratory distress syndrome was noted on a chest radiograph. A laryngeal anomaly and large tongue base were evident, which nearly obstructed the vocal cord. A physical examination revealed bushy eyebrows and synophrys, long curly eyelashes, long philtrum, downturned angles of the mouth and thin upper lips, cleft palate, micrognathia, excessive body hair, micromelia of both hands, flexion contracture of elbows and hypertonicity (Figs. 1, 2).
The patient had no relevant family history. The patient has two siblings born of a different father. They are healthy, morphologically normal children.
A brain sonogram revealed increased periventricular echogenicity. Computed tomography (CT) of the neck revealed no abnormality in upper airway except micrognathia. However, the patient had suffered from upper airway obstruction due to secretion from 1 month of age, which led to tracheostomy at 3 months of age. The patient exhibited feeding intolerance. Abdominal ultrasonography and upper GI series showed gastroesophageal reflux but no other abnormalities. Chromosome analysis showed a normal karyotype, 46, XY. The parents rejected their gene analysis. The patient had been hospitalized for 5 months due to recurrent sepsis and respiratory problems. At 5 months of age, the patient was discharged and did not visit for 5 months after discharge.
To analyze gene mutation, peripheral blood samples were obtained from the patient with the informed consent of the family. Genomic DNA was isolated from peripheral blood leukocytes using the Wizard Genomic DNA purification kit according to the manufacturer's instructions (Promega, Madison, WI, USA). Polymerase chain reaction (PCR) was performed using a Model 9600 thermal cycler (Applied Biosystems, Foster City, CA, USA), and direct sequencing of all coding exons and their flanking sequences of the NIPBL gene was accomplished using author-designed primer pairs (sequences available upon request) in an ABI Prism 3100 Genetic Analyzer with a BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems). Sequence variations were analyzed with reference to the wild type sequence (GenBank accession No. NM_002529) using the Sequencher program (Gene Codes, Ann Arbor, MI, USA).
Sequence analysis of the NIPBL gene revealed a heterozygous C→G transversion at nucleotide number 7178, which substituted the 2393rd serine to form a stop codon (Fig. 3). This is a novel mutation that has not reported elsewhere.
CdLS formerly described a child with similar features at autopsy in 1916, was first reported in 1933 by Cornelia de Lange (8). This syndrome is a multiple congenital anomaly characterized by distinctive facial features, hirsutism, upper limb malformations, gastroesophageal dysfunction, growth retardation, and neurodevelopmental delay. The facial features are characteristic and easily recognizable, with microcephaly, bushy eyebrows and synophrys, long and curly eyelashes, anteverted nares, long philtrum, thin lips, downturned angles of mouth, and micrognathia.
The feature of CdLS is expressed by a variable phenotype and ranges from mild to severe. Van Allen et al. (9) proposed a classification system based on the clinical variability. Type I or classic CdLS patients have the characteristic facial and skeletal changes. Type II or mild CdLS patients have similar facial features but minor skeletal abnormalities to those seen in Type I. Type III or phenoscopy CdLS have phenotypic manifestations of CdLS that are causally related to chromosomal aneuploidies or teratogenic exposures. Our patient exhibited characteristic facial features and severe skeletal symptoms as abscent forearm, which is compatable with Type I.
The incidence of CdLS is reported to be one in 10,000-100,000 births, although the exact incidence is unknown (10, 11). The etiology of CdLS is still not clear. Recently, heterozygous mutations in cohesin regulator and cohesin structural components have been linked to CdLS (3, 4, 12-15). Cohesin regulates sister chromatid cohesion during the mitotic cell cycle and is a multisubunit protein complex (12). In 2004, two independent groups reported that CdLS is caused by mutations in the NIPBL gene located on 5p13.2 (3, 4). The NIPBL gene is the human homologue of the Drosophila Nipped-B gene and belongs to the family of chromosomal adherins involved in chromatid cohesion processes and enhancer-promoter communications (13, 14). In addition, X-linked CdLS can arise from mutations in the SMC1A gene that encodes a subunit of the cohesion complex (15).
Approximately 60% of the probands with CdLS have heterozygous mutations in the NIPBL gene, while a smaller percent of cases exhibit mutations in the SMC1A or SMC3 cohesin subunit genes (3). There has been concern about genotype-phenotype correlation. Gillis et al. reported a significant differences between subjects with and without mutations in terms of the degree of growth retardation and developmental delay (16).
It has been demonstrated that mutations in NIPBL cause both mild and severe forms (9, 17). SMC1A and SMC3 mutations contribute to 5% of cases of CdLS and result in a consistently mild phenotype with absence of major structural anomalies (15, 18). However, another study reported that, in 53% of the mutations analyzed, 55% demonstrated a detectable mutation in NIPBL or SMC1A, and individuals with missense mutations in NIPBL and SMC1A presented with milder symptoms than exhibited with other mutations (19). In the latter study, two patients had nonsense mutations; both patients had a very small stature and had major malformations such as absent forearm and cleft palate.
Previous reports of NIPBL gene defects have identified 25 missense mutations, 19 nonsense mutations, 13 splicing mutations, 26 deletions mutations, and 16 insertion mutations (20). In the present patient, a novel and unreported mutation has been identified. To our knowledge, this is the first report of a CdLS confirmed NIPBL mutation in Korea. The present patient provides further evidence that more severe symptoms (very small stature, absent forearm and cleft palate) are associated with mutations, especially nonsense type, in NIPBL.
Diagnosis of CdLS is confirmed clinically, based on unique and easily recognizable features. Analysis of mutations in genes such as NIPBL and SMC1A gene are not necessary to diagnose CdLS. Indeed, the ability to analyze for mutation in the NIPBL, SMC1A and SMC3 genes does not currently exist in Korea. This capability would be advantageous, as it would facilitate family counseling and more definitive predictive prognosis.
Figures and Tables
Fig. 1
General appearance shows distinctive facial features, excessive body hair, micromelia of both hands, flexion contracture of elbows, and hypertonicity.
References
1. Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: a clinical review of 310 individuals. Am J Med Genet. 1993. 47:940–946.

2. Ireland M, Donnai D, Burn J. Brachmann-de Lange syndrome. Delineation of the clinical phenotype. Am J Med Genet. 1993. 47:959–964.

3. Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004. 36:631–635.

4. Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004. 36:636–641.

5. Mun YR, Ahn SI, Yang KS. De Lange syndrome. J Korean Med Assoc. 1967. 10:455–460.
6. Lee SH, Jang JW, Kim IS, Kim WD, Lee SG. A case of Cornelia de Lange syndrome with imperforate anus. J Korean Soc Neonatol. 2007. 14:253–257.
7. Kim IT, Park JW, Choi WC. A Korean case of Cornelia de Lange syndrome. Korean J Ophthalmol. 2005. 19:153–155.

8. Braddock SR, Lachman RS, Stoppenhagen CC, Carey JC, Ireland M, Moeschler JB, Cunniff C, Graham JM Jr. Radiological features in Brachmann-de Lange syndrome. Am J Med Genet. 1993. 47:1006–1013.

9. Van Allen MI, Filippi G, Siegel-Bartelt J, Yong SL, McGillivray B, Zuker RM, Smith CR, Magee JF, Ritchie S, Toi A, Reynolds JF. Clinical variability within Brachmann-de Lange syndrome: a proposed classification system. Am J Med Genet. 1993. 47:947–958.

10. Beck B. Epidermiology of Cornelia de Lange's syndrome. Acta Paediatr Scand. 1976. 65:631–638.
12. Liu J, Zhang Z, Bando M, Itoh T, Deardorff MA, Clark D, Kaur M, Tandy S, Kondoh T, Rappaport E, Spinner NB, Vega H, Jackson LG, Shirahige K, Krantz ID. Transcriptional dysregulation in NIPBL and cohesin mutant human cells. PLoS Biol. 2009. 7:e1000119.

13. Rollins RA, Morcillo P, Dorsett D. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics. 1999. 152:577–593.

14. Strachan T. Cornelia de Lange syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev. 2005. 15:258–264.

15. Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, Vezzoni P, Larizza L. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet. 2006. 38:528–530.

16. Gillis LA, McCallum J, Kaur M, DeScipio C, Yaeger D, Mariani A, Kline AD, Li HH, Devoto M, Jackson LG, Krantz ID. NIPBL mutational analysis in 120 individuals with Cornelia de Lange syndrome and evaluation of genotype phenotype correlations. Am J Hum Genet. 2004. 75:610–623.
17. Uzun H, Senses DA, Uluba M, Kocabay K. A newborn with Cornelia de Lange syndrome: a case report. Cases J. 2008. 1:329.

18. Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodríguez C, Arnedo M, Loeys B, Kline AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LS, Dorsett D, Krantz ID. Mutations in cohesion complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007. 80:485–494.
19. Kline AD, Grados M, Sponseller P, Levy HP, Blagowidow N, Schoedel C, Rampolla J, Clemens DK, Krantz I, Kimball A, Pichard C, Tuchman D. Natural history of aging in Cornelia de Lange syndrome. Am J Med Genet C Semin Med Genet. 2007. 145C:248–260.

20. Su PH, Yu JS, Chen SJ, Chen JY. Identification of a novel mutation and polymorphic change in the NIPBL gene of subjects with Cornelia de Lange syndrome. Genes Genomics. 2008. 30:253–260.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download