Abstract
Despite advances in neuroimaging and neurosurgical treatment modalities, spinal epidural abscess remains a challenging problem. Early diagnosis is often difficult and treatment is always delayed. Spinal epidural abscess usually develops in patients with predisposing factors such as IV drug abuse, senillity, diabetes mellitus, spinal attempts, alcoholism, immunosuppression, liver diseases and catheterizations. It is rarely seen in cervical region. A successful treatment is only possible with early diagnosis and accurate surgical and medical treatment. Optimal management is unclear and morbidity and mortality are significant. We present two adult haemodialysis patients with end-stage renal insufficiency who developed cervical epidural abscess following central venous catheter placement. Early surgical intervention is mandatory in cases those have progressive neurological deficit and spinal deformity, and this is also increases the success rate of medical therapy.
Nonspecific pyogenic spinal epidural abscess (SEA) is an uncommon entity, especially in cervical region, but its clinical importance overshadows its rarity (1-6). The overall frequency has been reported to be between 0.2 and 2 per 10,000 hospitalized patients (5, 7-10). Because of the indistinct symptoms, it causes the patient to become neurologically worse in short span of time (2, 6, 8, 11-13). A successful treatment is possible with early recognition and beginning appropriate treatment immediately (5-7, 9, 10, 14). In the presence of the predisposing factors, such as IV drug abuse, senility, diabetes, mellitus, alcoholism, immunosuppression, liver diseases, renal failure or surgical attempts such as spinal/central venous catheterization, indistinct symptoms of the patient such as dorsal-lumbar-neck pain or subfebril fever must be examined seriously (5, 8, 10, 11, 13). We report two cases of cervical epidural abscesses in haemodialysis patients and discuss clinical features, role of neuroimaging techniques for rapid diagnosis and guidelines for their medical and surgical management.
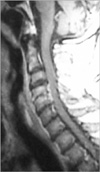
A 66-yr-old male patient was in haemodialysis program for 1 yr due to end-stage renal insufficiency. After a central venous catheterization had been applied three weeks ago in another hospital, he had been referred to our clinic with severe neck pain, high fever and rapid progressive quadriparesis. In the physical examination, he was febril (37.2℃) but no neck stiffness was present. In the neurological examination, he was quadriparetic (motor strength was proximally 3/5, distally 1/5 in upper extremities, 1/5 in lower extremities). Babinski, Hofmann, and clonus reflexes were bilaterally positive and he was hypoesthetic below C4 dermatome. Laboratory tests were normal. There were no any specific findings for osteomyelitis in cervical roentgenograms. Postgadolinium magnetic resonance (MR) revealed an epidural abscess at C4-5 level (Fig. 1). At surgery, via a posterior midline approach, we performed hemilaminectomy to C4 and C5 levels and partial facetectomy and debridement of infective tissues. The cultures yielded no organism. Postoperative period of the patient was uneventful and he transferred to infection diseases clinic to have the antimicrobial therapy (vancomycin 2 gr/day and ceftriaxone 2 gr/day). In follow-ups, his spasticity and quadriparesis recovered gradually to 3/5 in upper extremities and 2/5 in lower limbs.
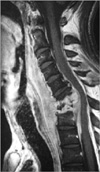
A 45-yr-old male patient was in haemodialysis program for 3 yr due to end-stage renal insufficiency. He had a history of two operations for peritonitis because of performing peritoneal dialysis before. After a central venous catheterization had been applied one month ago, he had been referred to our clinic with severe neck pain, high fever and acute progressive quadriparesis. In his neurological examination, he was quadriparetic (motor strength was promixally 3-4/5, distally 1-2/5 in upper extremities and 3/5 in the lower extremities). He complained hypoesthesis below C6 dermatome. Babinski, Hofmann, and clonus reflexes were bilaterally positive. The peripheral white blood cell (WBC) count was 13.8×109/L, erythrocyte sedimentation rate (ESR) was 136 mm/hr and creactive protein (CRP) level was 52.5 mg/dL, and the other laboratory findings were normal. MR images revealed an epidural abscess, spondylodiscitis and anterior kyphotic deformity with cord compression in C5 to C7 levels (Fig. 2). At surgery, via anterior approach, total corpectomies to C5, C6, and C7, and partial corpectomies to C4 and T1 bodies were performed. After drainage of the abscess located epidurally and debridement of necrotic tissues, plate fusion procedure was performed by the autograft taken from the patient's fibula to the corpectomy area. The pathogens isolated from the pus culture were Staphylococcus aureus and Pseudomonas aeruginosa which were resistant to methicillin and he transferred to infection diseases clinic to have the antimicrobial therapy (tazocin 7.5 gr/day, targocid 400 mg/72 hr). Postoperative period was uneventful and after 3 weeks he mobilized with SOMI corset. In follow-ups, his spasticity and quadriparesis recovered gradually to 3-4/5 in upper extremity and 3/5 in the lower extremity.
SEA occurs primarily in individuals over 30 yr of age. However, published series with more than ten patients report differing average ages for affected patients (4, 6, 8, 10, 13). The colonization of the spinal epidural space by micro-organisms may occur hematologenously or by contiguous spread from neighboring structures. Infection due to hematogenous spread may originate from all types of extraspinal infection leading to a persistent or temporary bacteremia. All invasive procedures can lead to the introduction of bacteria into the blood stream with resultant SEA. In addition, many patients have risk factors that may favor the development of SEA (4, 6, 8, 10, 13). It is also reported after central venous catheterization in patients on maintenance haemodialysis (6, 10, 13-16). The preferential thoracic or lumbar localization of SEA is likely related to the greater extension of the epidural space in the thoraco-lumbar segment of the vertebral column and to the well-developed extradural venous plexus in this region (3, 4, 17-20). In the present cases, the reason of cervical localization might be the using of internal jugular veins.
In the clinical symptoms, following the initial stage of severe pain associated with local tenderness in the area of the spinal column, the second stage is dominated by signs of spinal irritation. These manifestations include Laseque's, Kernig's and Lhermitte's signs, Brudzinski's reflex and neck stiffness (5). In the third stage, initial neurologic deficits are observed such as weakness of the voluntary musculature or fecal or urinary incontinence. Importantly, the transition to the terminal stage with paralysis can occur very quickly (5). In addition to neurological findings, patients with SEA may also develop fever in the initial stage due to inflammation associated with the abscess. This is accompanied by an elevated ESR and leukocytosis (3-5, 8, 11).
Radiological investigations have been the mainstay of diagnosis for patients with SEA. However, the most important step in diagnosing SEA is consideration of the entity. So, delay in the diagnosis of this entity, some manifest neurological deficits are seen on approximately 60% of the patients, like as in our cases (2-4, 7, 11, 12, 20). Before the era of computerized tomography (CT) and magnetic resonance imaging (MRI), the only imaging modalities of use in diagnosis were conventional radiographs and myelography. However, conventional radiography of the vertebral column is not necessarily reliable for SEA. Only after erosion or sclerotic changes of bony structures and these changes are to be expected only after several weeks of chronic SEA (8). In contrast to CT, MRI is able to form multiplanar images with high contrast among soft-tissue structures and without bone artifacts. In a study, it was reported a sensitivity of 95% in MRI for the definitive diagnosis of SEA (17). MR findings are similar with acute spondylodiscitis. It reveals low signal intensity in vertebra corpus in T-1 weighted images and destruction in cortex and high signal intensity at effected corpus in T-2 weighted images (4, 17) (Fig. 1, 2). The use of gadolinium in MRI allows better delineation of SEA from contiguous structures.
A wide range of microorganisms can cause SEA, and isolation of the aetiologic agent, particularly from blood culture, bone biopsies, or aspirates from bone or paravertebral abscess, is necessary in order to secure appropriate antimicrobial therapy. The easiest and the least invasive method to obtain the agent is from blood and its isolation rate is reported as 68% in cultures of blood (13). Another method is fine-needle biopsy guided with CT or fluoroscope which success rate varies from 35% to 63% (13). Neverthless, the most successful route in obtaining the pathogen is via open surgery (3, 4, 8, 11).
SEA is primarily a bacterial infection (1, 4-6, 8, 10-13, 18). The few reported cases of fungal infections were seen mainly in immunocompromised patients and individual cases of SEA due to parasites have been reported from certain geographic regions. Staphylococcus aureus was described as the principle etiologic agent of SEA in the international literature. Gram negative bacteria may be more common in cases of SEA in drug users.
Although conservative treatment modalities have occasionally been reported to be successful, in our opinion, surgical intervention is the method of choice and is generally performed with pre- and postoperative administration of antibiotics. Conservative method is only possible for cases without any neurological deficits in early periods and together with their close neurological follow ups (1, 8, 19). The most commonly used operative procedure was laminectomy, via posterior approach. Intraoperative sonography is especially useful in the surveillance of surgical decompression of anteriorly located extensions of the abscess. Anterior decompression is necessary for SEA in the anterior segment of the epidural space and this operation is usually combined with anterior corpectomy. The choice of surgical approach in the cases which have manifest neurological deficit, as in our both cases, provides decompression of spinal cord, debridement of infected/necrotic material, drainage of abscess and correction of spinal deformity in one session. Although the issue of appropriate surgical approach-anterior versus posterior-in SEA is controversial, we think it should be individualized according to location of the abscess and its own features. The second main problem is application of a foreign material into the infected area and this is also controversial. However, in some conditions, as in our second case, because of extensive bone resections, foreign material implantation and reconstruction will be compulsory and today wide spectrum antibacterials can make it possible (3, 4, 8, 19). The antibacterial treatment policy should fulfill these criteria: 1) efficacy against Staphylococcus aureus, the most common cause of SEA, 2) low toxicity to enable treatment over several weeks, and 3) the ability to penetrate bony tissues, as also is necessary in treating spondylodiscitis. A standard antibiotic treatment regimen cannot be derived from published series. The choice of antibiotics is based on the preferences of the clinician and the institution, resistance testing, and the country of treatment. The duration of antibiotic administration is up to 12 weeks but usually 4 to 6 weeks. Antibiotics should be initially given intravenously but may be continued with oral formulations.
In conclusion, it is known that optimal management is unclear in SEA, and morbidity and mortality are still significant in spite of modern diagnostic and treatment opportunities. We present two adult haemodialysis patients who developed cervical epidural abscess with end-stage renal insufficiency following central venous catheter placement. Early surgical intervention is mandatory in cases those have progressive neurological deficit and spinal deformity, and this is also increases the success rate of medical therapy.
Figures and Tables
References
1. Ahl T, Hedstrom M, von Heijne A, Hammers Stiernstedt S. Acute spinal epidural abscess without concurrent spondylodiscitis. Successful closed treatment in 10 cases. Acta Orthop Scand. 1999. 70:199–202.

2. Kimura T, Hashizume K, Tanaka T, Yonemasu Y. A case of acute cervical epidural abscess. No To Shinkei. 1997. 49:359–363.
3. Lu CH, Chang WN, Lui CC, Lui CC, Lee PY, Chang HW. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg. 2002. 104:306–310.

4. Mann S, Schutze M, Sola S, Piek J. Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients. Neurosurg Focus. 2004. 17:E3.

5. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a metaanalysis of 915 patients. Neurosurg Rev. 2000. 23:175–204.

6. Sugimoto M, Tamaki N, Nagashima T, Kokunai T, Tomita H, Sakagami Y. Cervical spinal epidural abscess caused by methicillin-resistant Staphylococcus aureus (MRSA). No Shinkei Geka. 1994. 22:973–976.
7. Khan SH, Hussain MS, Griebel RW, Hattingh S. Title comparison of primary and secondary spinal epidural abscesses: a retrospective analysis of 29 cases. Surg Neurol. 2003. 59:28–33.
8. Rigamonti D, Liem L, Sampath P, Knoller N, Namaguchi Y, Schreibman DL, Sloan MA, Wolf A, Zeidman S. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999. 52:189–196.

9. Sampath P, Rigamonti D. Spinal epidural abscess: a review of epidemiology, diagnosis, and treatment. J Spinal Disord. 1999. 12:89–93.
10. Valero R, Castaneda O, de Francisco AL, Pinera C, Rodrigo E, Arias M. Clinical suspicion of vertebral osteomielitis: back pain in patients with hemodyalisis by catheter related infection. Nefrologia. 2004. 24:583–588.
11. Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987-97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998. 30:147–151.
12. Fieseler HG, Kumm D, Braun M. An important differential diagnosis for persistent back pain. Schmerz. 2001. 15:110–115.
13. Priest DH, Peacock JE Jr. Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J. 2005. 98:854–862.

14. Kolmos HJ. Spinal epidural abscess in patients on maintenance haemodialysis: a presentation of two cases. Int Urol Nephrol. 1979. 11:249–253.

15. Philipneri M, Al-Aly Z, Amin K, Gellens ME, Bastani B. Routine replacement of tunneled, cuffed, hemodialysis catheters eliminates paraspinal/vertebral infections in patients with catheter-associated bacteremia. Am J Nephrol. 2003. 23:202–207.

16. Torda AJ, Gottlieb T, Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis. 1995. 20:320–328.

17. Lang IM, Hughes DG, Jenkins JP, St Clair Forbes W, McKenna F. MR imaging appearances of cervical epidural abscess. Clin Radiol. 1996. 50:466–471.

18. Muffolerro AJ, Nader R, Westmark RM, Nauta HJ, Garges KJ, Hadjipavlou AG. Hematogenous pyogenic facet joint infection of the subaxial cervical spine: a report of two cases and review of the literature. J Neurosurg. 2001. 95:1 suppl. 135–138.
19. Lam KS, Pande KC, Mehdian H. Surgical decompression: a life-saving procedure for an extensive spinal epidural abscess. Eur Spine J. 1997. 6:332–335.

20. Tang HJ, Lin HJ, Liu YC, Li CM. Spinal epidural abscess experience with 46 patients and evaluation of prognostic factors. J Infection. 2002. 45:76–81.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download