Abstract
This study was conducted to evaluate the immunogenicity and safety of diphtheria-tetanus (Td) vaccine in adults over 40 yr old who had never received a diphtheria-tetanus-pertussis (DTP) vaccination. A total of 242 subject completed three-doses of Td vaccination and subsequent assays for immunogenicity. Before vaccination, 33.9% and 96.7% participants showed antibody levels of diphtheria and tetanus, respectively, which were below protective level (<0.1 U/mL). After the first dose of Td vaccine, 92.6% and 77.6% of subjects gained protective antibody concentrations (≥0.1 U/mL) for diphtheria and tetanus, with an increase to 99.6% and 100% after the third dose. Local and systemic adverse events occurred in 37.9% and 15.5% of the subjects. No serious adverse event requiring an unscheduled hospital visit occurred. In conclusion, three-doses of Td vaccination to unimmunized adults are safe and effective in inducing protective immunity against diphtheria and tetanus.
Diphtheria and tetanus can be only prevented by vaccination because immunity against these diseases is rarely acquired, even by natural infections (1, 2). To maintain long-term protective immunity against diphtheria and tetanus, booster immunization is essential for adolescents and adults (3, 4). In most countries, booster immunization of diphtheria and tetanus is recommended to be performed every 10 yr in people who had completed DTP vaccination at 4-5 yr old. Booster Td vaccine is recommended to start at the age of 11-12 yr (5, 6).
In Korea, the diphtheria-tetanus-whole cell pertussis (DTwP) vaccine was introduced in 1958. Therefore, those who were born before 1958 had never been vaccinated with either DTwP or diphtheria-tetanus-acellular pertussis (DTaP) vaccine introduced in 1982. In Korea, only 1-16 cases of tetanus have been reported since 2000; and not a single case of diphtheria has been reported since 1987. However, seroepidemologic studies revealed that there were groups vulnerable to diphtheria and tetanus (7-9). More than 90% of the study population had antibody levels against tetanus and about 45% had antibody levels against diphtheria, with concentrations of less than 0.1 U/mL among Korean adults over the age of 40 yr. Also the mean antibody levels of diphtheria and tetanus were inversely related with age (8, 9). These findings, together with the large epidemic of diphtheria in the former Soviet Union in the 1990s and the prevalent diphtheria in Asian countries, urged us an introduction of Td vaccine to our country (10-12).
After an amendment to the School Health Law in 2005, middle schools were stipulated to confirm Td vaccination upon their admission. The vaccination rate is now approximately 40% in 11-12 yr old (7). The effectiveness and safety of booster injection of Td vaccine in pre-adolescent and adolescent were reported in Korea (13, 14). But this vaccine is rarely done in adults, except for tetanus prophylaxis in cases of traumatic injuries.
We conducted this study to assess the immunogenicity and safety of three-doses of Td vaccination according to age, sex, and vaccine dose, in Korean adults aged 40 yr or older who had had an opportunity to receive neither DPT nor Td vaccines, in order to formulate concrete evidence of efficacy of Td vaccine for adults.
Adults (40 yr or older), who had not received DPT or Td vaccination and who were willing to participate in this study, were enrolled. People who had mild systemic diseases such as diabetes mellitus or hypertension which were controlled by oral medications and cared for in outpatients clinics were allowed to be included. The study was conducted as a multicenter, non-randomized, open label phase 4 study from August 2007 to December 2008 in five hospitals in Korea.
After agreement, history taking and physical examinations were done. A complete blood cell count, blood chemistry including liver function, kidney function, and glucose levels were checked.
A 0.5 mL dose of Td vaccine (SK Chemical Td-pur®, Seoul, Korea), containing 1.5 limes flocculation unit (Lf) diphtheria toxoid and 5 Lf tetanus toxoid with 1.5 mg aluminum hydroxide was given three times intramuscularly into the deltoid, via prefilled syringes with 25-gauge needle of length 2.54 cm. The first dose of the vaccine was administered immediately after screening and enrollment, the second dose was injected 4 weeks after the first dose, and the third dose was administered 5 to 6 months after the second dose.
Blood samples (5 mL) were collected before injection, 4 weeks after the first injection and 4 weeks after the third injection. Serum samples were kept at -70℃ until analysis. Two different kinds of enzymes linked immunosorbent assay kits (kit number RE56191 for diphtheria and RE56901 for tetanus) from same company (IBL, Hamburg, Germany) were used to determine the serum levels of antibodies to diphtheria and tetanus, according to manufacturer's instructions. Briefly, 100 µL of each standard and diluted test serum were put into the wells coated with either the diphtheria or tetanus antigens. The plate was covered and incubated 60 min at room temperature. After discarding the incubated solution, the plate was washed three times with 300 µL of diluted wash buffer. A total of 100 µL of enzyme conjugate was put into each well and incubated for 30 min at room temperature. After discarding the incubation solution, the plate was washed three times with 300 µL of diluted wash buffer. After the addition of substrate and stop solution, 100 µL of TMB (3,3',5,5'-tetramethylbenzidine base) substrate solution was put into each well. After incubating 20 min at room temperature in the dark, the substrate reaction was stopped by adding 100 µL of TMB stop solution into each well. Optical density was measured with a photometer at 450 nm. The levels of antibodies in the samples were read by the standard curve obtained from the standard samples. Antibody levels ≥0.1 U/mL against diphtheria or tetanus were considered indicative of seroprotection.
Subjects were given diary cards and asked to record any local adverse events (itching, pain, redness, and swelling at the injection site) or systemic adverse events (fatigue/malaise, fever, gastrointestinal problems, headache, and respiratory symptoms) that occurred during the 2-week (days 0-14) follow-up period after the vaccination. All subjects were monitored for an additional 2-weeks (days 15-28), as a safety follow-up period, for unscheduled hospital visits and serious adverse events (death, life threatening event, hospitalization, any event leading to sequelae). Unsolicited adverse events and serious adverse events that occurred during the 4-week (days 0-28) study period were also recorded. Causal relationships between adverse events and the vaccination were evaluated by the investigators.
Geometric mean concentration (GMC) and the seroprotection rate were calculated with 95% confidence interval (CI) for each antibody measured at each blood sampling time point. The mean of changes in antibody concentrations between pre- and post-vaccination was analyzed with paired t-test. The difference of antibody concentrations by age and gender was analyzed with ANOVA or t-test at each blood sampling time point.
All adverse events (solicited and unsolicited) recorded in report forms were classified into the system organ class, according to preferred terms. The incidence rate of adverse events and its 95% CI were estimated and analyzed using chi-squared-test or Fisher's exact test.
The protocol and associated documents were reviewed and approved by the ethics committee of each study hospital. The numbers of the approvement documents of each hospital are listed as follows: Incheon St. Mary's Hospital (OCMC07MI088), Yeouido St. Mary's Hospital (SCMC07MI014), Bucheon Soonchunhyang University Hospital (07-32), National Health Insurance Company Ilsan Hospital (2007-41), and Kangdong Sacred Heart Hospital (08-20). Written informed consent was obtained from all subjects.
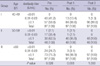
A total of 290 subjects were enrolled. During the follow-up period, 37 subjects did not complete the three-times injections of Td vaccine and 11 subjects did not give the three blood samples. Demographic characteristics of subjects are described in Table 1. Subjects were classified into three groups according to age.
Two hundred and forty-two subjects completed the three doses of vaccinations and blood samplings for assays. Prior to vaccination (Pre), GMCs for anti-diphtheria and anti-tetanus antibody were 0.3489±0.5255 U/mL and 0.0311±0.0629 U/mL, respectively. Eighty-two of 242 (33.9%) and 234 of 242 (96.7%) participants showed antibody levels of diphtheria and tetanus of less than 0.1 U/mL, respectively. After the first dose of Td vaccination (Post 1), 224 (92.6%) and 188 (77.6%) participants showed antibody levels of more than 0.1 U/mL for diphtheria and tetanus, respectively. After the third dose vaccination (Post 3), 241 (99.6%) and 242 (100%) participants revealed antibody levels of more than 0.1 U/mL for diphtheria and tetanus, respectively (Fig. 1). GMCs for anti-diphtheria antibody and anti-tetanus antibody were 1.8210±1.2820 U/mL and 1.3159±2.1261 U/mL each, after the first dose; and 2.0629±0.9351 U/mL and 4.4267±3.0746 U/mL each, after the third dose.
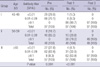
There was no difference according to age groups with regard to gaining anti-diphtheria and anti-tetanus antibodies after three-doses of vaccination. There was, however, a significant difference in antibody formation found according to the age group, after the first dose of vaccination. The older age groups (group II and III) showed a higher antibody levels for diphtheria (P=0.006). In contrast, the older age groups showed a lower antibody levels for tetanus compared to the younger age (group I) (P=0.001) (Tables 2, 3).
Analysis of seroprevalance among subjects separated by sex revealed a significantly higher proportion of pre-vaccination antibody levels ≥0.1 IU/mL to diphtheria (P=0.004) and tetanus (P<0.001) among men compared to women. Post-vaccination seroprevalence did not show significant difference according to sex (diphtheria, P=0.845 and 1.000 after first and third dose; tetanus, P=0.179 and 1.000 after first and third dose of Td vaccine).
Although 66.1% of subjects showed antibody levels to diphtheria ≥1.0 U/mL after one-dose of Td vaccination, only 28.5% of subjects showed antibody levels ≥1.0 U/mL to tetanus. After three-doses of Td vaccination, 83.1% and 93.8% of subjects achieved antibody levels ≥1.0 U/mL to diphtheria and tetanus, respectively (Fig. 1).
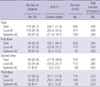
During the 7-month follow-up period after the vaccination, solicited local adverse events occurred in 110 (37.9%) subjects. Solicited systemic adverse events occurred in 45 (15.5%) subjects (Table 4). Pain at the injection site was the most common local adverse event, and malaise was the most common systemic adverse event. Febrile episodes were observed in less than 3% of subjects after each vaccination. All of the adverse events resolved within 7 days without specific medical care, and no serious adverse event requiring an unscheduled hospital visit occurred. The rate of adverse events according to vaccination dose were 31.0%, 22.9%, and 26.5% after each injection and there was no statistical difference according to vaccination frequency (P=0.09). The differences in the rate of adverse events according to sex and age groups were not evaluated.
This study was conducted to evaluate the immunogenicity and safety of the Td vaccine in Korean adults aged 40 yr old or older who had not received primary vaccination against diphtheria and tetanus. The results showed satisfactory immunogenicity and acceptable adverse events in this population.
In Korea, DTwP vaccine was introduced in 1958 and DTaP vaccine has been used for primary and booster immunizations since 1982 (7-9). A DTaP immunization coverage of more than 90% has resulted in a marked decrease in the prevalence of diphtheria and neonatal tetanus, and no case of diphtheria has been reported since 1987 (7, 8). There was a temporary decrease in DTaP coverage because of misunderstood contraindications of the vaccine and exaggerated reports of unconfirmed adverse events. Under these circumstances, around 10 cases of tetanus were reported annually in Korea (15) and one case of polyneuropathy assumed to be caused by a diphtheria infection was reported (16). Because immunity against diphtheria and tetanus wanes in the absence of routine administration of booster vaccinations among adolescents and adults, cohorts of susceptible people increase. A previous seroepidemiologic Korean study reported that 48-89% of studied subjects had antibody against diphtheria of less than 0.1 U/mL according to age groups. Subjects who were 20-49 yr old showed lower GMCs compared to subjects older than 50 yr old. The study also showed that about 90% of subjects over 40 yr of age had antibodies against tetanus of less than 0.1 U/mL, with 20% of subjects in this age group less than 0.01 U/mL (8, 9).
Seroepidemiologic evaluation of pre-vaccination state revealed again that 33.9% and 96.7% of participants did not have protective immunity against diphtheria and tetanus, respectively. One-dose of Td vaccination provided protective immunity in 92.6% and 77.6% of subjects against diphtheria and tetanus, respectively. One confusing results was that the participants in the older age groups tended to achieve higher antibody levels against diphtheria. This result was caused by a greater proportion of the older age groups that were already maintaining a higher levels of antibodies against diphtheria before vaccination. This phenomenon was also observed in a previous domestic study. Older people maintain a higher antibody levels against diphtheria possibly because of long-term exposure to diphtheria before the vaccine era, which thereby stimulated natural immunity (8).
Three-doses of Td vaccination provided acceptable protective immunity against diphtheria and tetanus in all participants. These results are compatible to previous studies which revealed that more doses of Td vaccination could provide more protective immunity and prevent development of severe diseases, especially for diphtheria (17-19). These studies provided valuable data about the effect of Td vaccine on diphtheria, especially after a large outbreak in the former Soviet Union, but the effect on tetanus was not evaluated.
As there have been no confirmed cases of diphtheria in Korea since 1987, a greater emphasis should be placed on tetanus prevention. Although a few cases of tetanus were reported in this country, a previous report analyzing 17 adult cases of tetanus gave information about the epidemiology of tetanus in Korea and led to the acknowledgement of the importance of Td vaccination in adults. In this study, the mean age of tetanus patients was 63 yr old (range, 29-87) and 88.2% of the patients received neither the primary vaccination nor decennial booster vaccination. The fatality rate was 23.5% (15).
Our study strongly suggests that Td vaccination is essential to Korean residents over 40 yr of age who never received a primary DTaP vaccine and booster Td vaccine. Additionally, to achieve sufficient protective immunity to tetanus, at least three-doses of Td vaccination is inevitable. Three-doses of Td vaccination provided 100% protection against tetanus, even in older people. Furthermore, it guaranteed long-term protective immunity (GMC ≥1.0 U/mL) in 94% of subjects against tetanus. These results were supported by a Brazilian study: Weckx et al. assessed the immunogenicity of one dose of Td vaccine to elderly subjects (median age 84 yr old). Before vaccination, 18% of the individuals were susceptible (GMC <0.01 U/mL) to diphtheria and 94% were susceptible to tetanus. After one dose of Td vaccine, 78% became immune (GMC ≥0.1 U/mL) to diphtheria, but 79% remained still susceptible to tetanus. They concluded that complete vaccination series appears to be necessary for the prevention of tetanus to elderly subjects (20).
Adverse events following vaccination developed in 41.7% (local adverse events 38% and systemic adverse events 16%) of subjects. The incidence is markedly less compared to the study performed in pre-adolescents and adolescents with the same Td vaccine in Korea. More local (68-83%) and systemic (25-32%) adverse events were reported in that study. This discrepancy might be due to active reporting of adverse events by parents in that study (13, 14). A study performed by Khetsuriani et al. (19) reported only a 5.3% adverse reaction rate from 248 participants. It is generally accepted that the Td vaccine causes fewer and milder adverse events than DTaP vaccine (21, 22).
Most adverse events reported during this study period were mild and resolved spontaneously within 7 days, without special treatment such as hospitalization. There were no serious adverse events, such as Arthus reaction or anaphylaxis, requiring an unscheduled hospital visit.
Although two-thirds of the participants were women in this study, there was no difference in gaining immunity to diphtheria and tetanus according to sex. This result agrees with the study performed by Khetsuiani et al. (19) in the Republic of Georgia, with the Td vaccine containing less than 5 Lf of toxoids produced by an Indian company. According to this result, we can assume that sex is not a limiting factor to gaining immunity to diphtheria and tetanus by Td vaccine, regardless of vaccine manufacturing company.
In conclusion, we could demonstrate the effectiveness and safety of three-doses of Td vaccination in inducing an adequate immune response against diphtheria and tetanus, regardless of age and sex.
Figures and Tables
Fig. 1
Distribution of antibody titers pre- and post-vaccination of Td vaccine.
(A) diphtheria; (B) tetanus; Pre, before first dose of vaccination; Post 1, 4 weeks after first dose of vaccination; Post 3, 4 weeks after third dose of vaccination.

References
1. Turner TB, Velasco-Joven EA, Prudovsky S. Studies on the prophylaxis and treatment of tetanus. II. Studies pertaining to treatment. Bull Johns Hopkins Hosp. 1958. 102:71–84.
2. Cain HD, Falco FG. Recurrent tetanus. Calif Med. 1962. 97:31–33.
3. Vitek CR, Wharton M. Plotkin SA, Orenstein WA, Offit PA, editors. Diphtheria toxoid. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders Co;139–156.

4. Wassilak SG, Roper MH, Kretsinger K, Orenstein WA. Plotkin SA, Orenstein WA, Offit PA, editors. Tetanus toxoid. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders Co;805–840.

5. Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995. 332:761–766.

6. Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, Srivastava PU, Moran JS, Schwartz B, Murphy TV. Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006. 55:RR-3. 1–34.

7. Kang JH. The need of Td vaccination according to the changes of tetanus and diphtheria Immunity. J Korean Med Assoc. 2008. 51:127–136.

8. Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Baek SY, Hong SH, Min HK. Age related seroepidemiological study of diphtheria among Koreans. Korean J Infect Dis. 2000. 32:1–7.
9. Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Ban SJ, Hong SH, Cho DH, Lee SH. Age related serosurvey of immunity to tetanus in Korean populations. Korean J Infect Dis. 2001. 33:104–111.
10. Galazka AM, Robertson SE, Oblapenko GP. Resurgence of diphtheria. Eur J Epidemiol. 1995. 11:95–105.

11. Hardy IR, Dittmann S, Sutter RW. Current situation and control strategies for resurgence of diphtheria in the newly independent states of former Soviet Union. Lancet. 1996. 347:1739–1744.
12. MacGregor RR. Mandell GL, Bennett JE, Dolin P, editors. Corynebacterium diphtheriae. Principles and Practice of Infectious Diseases. 2009. 7th ed. Philadelphia: Elsevier;2687–2693.

13. Lee SY, Kwak GY, Mok HR, Kim JH, Hur JK, Lee KI, Park JS, Ma SH, Kim HM, Kang JH. The immunogenicity and reactogenicity of Td booster vaccination in Korean preadolescence, aged with 11-12 years old. Korean J Pediatr. 2008. 51:1185–1190.
14. Lee SY, Kwak GY, Nam CH, Kim JH, Hur JK, Lee KY, Park JS, Kim HM, Kang JH. Immunogenicity and safety of diphtheria-tetanus vaccine in pre-adolescent and adolescent South Koreans. Vaccine. 2009. 27:3209–3212.

15. Shin DH, Yu HS, Park JH, Shin JH, Kim SJ. Recently occurring adult tetanus in Korea: emphasis on immunization and awareness of tetanus. J Korean Med Sci. 2003. 18:11–16.

16. Yun YH, Park HJ, Yu SW, Kwon SB, Minn YK, Cho SJ, Kwon KH. A case of polyneuropathy suggesting diphtheritic neuropathy. J Korean Neurol Assoc. 2005. 23:288–289.
17. Brennan M, Vitek C, Strebel P, Wattigney W, Bisgard K, Brisgalov S, Bragina V, Pyanikh V, Wharton M. How many doses of diphtheria toxoid are required for protection in adults? Results of a case-control study among 40- to 49-year-old adults in the Russian Federation. J Infect Dis. 2000. 181:Suppl 1. S193–S196.

18. Sutter RW, Hardy IR, Kozlova IA, Tchoudnaia LM, Gluskevich TG, Marievsky V, Deforest A, Wharton M. Immunogenicity of tetanus-diphtheria (Td) among Ukrainian adults: Implications for diphtheria control in the newly independent states of the former Soviet Union. J Infect Dis. 2000. 181:Suppl 1. S197–S202.
19. Khetsuriani N, Music S, Deforest A, Sutter RW. Evaluation of a single dose of diphtheria toxoid among adults in the Republic of Georgia, 1995: immunogenicity and adverse reactions. J Infect Dis. 2000. 181:Suppl 1. S208–S212.

20. Weckx LY, Divino-Goes K, Lihama DM, Carraro E, Bellei N, Granato CF, Moraes-Pinto MI. Effect of a single tetanus-diphtheria vaccine dose on the immunity of elderly people in S㯠Paulo, Brazil. Braz J Med Biol Res. 2006. 39:519–523.
21. Vilella A, Dal-Re R, Simo D, García-Corbeira P, Diego P, Bayas JM. Reactogenicity profile of tetanus-diphtheria (adult-type) vaccine: results of a naturalistic study performed at an adult vaccination center. J Clin Pharmacol. 2000. 40:1267–1273.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download