Abstract
Anti-erythropoietin antibodies usually cross-react with all kinds of recombinant erythropoietins; therefore, erythropoiesis-stimulating agent (ESA)-induced pure red-cell aplasia (PRCA) is not rescued by different ESAs. Here, we present a case of ESA-induced PRCA in a 36-yr-old woman with chronic kidney disease, whose anemic condition improved following reintroduction of darbepoetin-α. The patient developed progressive, severe anemia after the use of erythropoietin-α. As the anemia did not improve after the administration of either other erythropoietin-α products or erythropoietin-β, all ESAs were discontinued. Oxymetholone therapy failed to improve the transfusion-dependent anemia and a rechallenge with ESAs continuously failed to obtain a sustained response. However, her anemia improved following reintroduction of darbepoetin-α at 3 yr after the initial diagnosis. Interestingly, anti-erythropoietin antibodies were still detectable, although their concentration was too low for titration. In conclusion, darbepoetin-α can improve ESA-induced PRCA when the anti-erythropoietin antibody titer declines and its neutralizing capacity is lost.
Pure red-cell aplasia (PRCA) is a disorder of erythropoiesis that leads to sudden-onset, progressive and severe anemia. Since 1998, there have been cases of recombinant human erythropoietin (rEPO) antibody-associated PRCA in patients with chronic kidney disease who receive subcutaneous treatment with rEPOs. In general, patients developing erythropoiesis-stimulating agent (ESA)-induced PRCA should not be treated with another ESA, because anti-EPO antibodies will certainly cross-react with the ESA and can induce systemic adverse reactions (1, 2). However, some case reports have described patients with ESA-induced PRCA who recovered responsiveness to the same or different ESA after immunosuppressive therapy. A rechallenge with the same or another ESA has been proposed after patients become free off the antibodies following immunosuppressive therapy or renal transplantation (3, 4). Herein, we report a case of ESA-induced PRCA in a 36-yr-old woman with chronic kidney disease caused by immunoglobulin A nephropathy (5), whose condition improved after reintroduction of darbepoetin-α when the anti-EPO antibody titer declined without further immunosuppression.
A 36-yr-old female patient was admitted for severe anemia in July 2002. She had been diagnosed with chronic kidney disease caused by immunoglobulin A (IgA) nephropathy. In October 2000, she began to receive rEPO therapy with Epokine (CJ Corp, Seoul, Korea), an EPO-α product, at a dose of 4,000 IU/week on subcutaneous (SC) route for anemia. Her hemoglobin (Hb) level was maintained at 10-12 g/dL before hemodialysis. In January 2002, she was started on hemodialysis, and her Hb level was maintained at 8-10 g/dL under EPO-α treatment at a dose of 3,000-6,000 IU/week. Eleven months after the start of hemodialysis, her Hb level dropped to 5.3 g/dL, although she was treated with rEPO-α at a dose of 12,000 IU/week. Even with the cumulative ESA dose of 224,000 IU over 26 months, her anemia did not improve. Therefore, she was transfused with two units of packed red blood cells every three weeks to maintain her Hb level despite the ESA treatment (12,000-15,000 IU/week). Meanwhile, she received three types of rEPO-α products (Epokine, Espogen [LG Life Sciences, Seoul, Korea], and Eporon [Dong-A Pharmaceutical Co., Ltd., Seoul, Korea]) and one rEPO-β (Recormon [Roche, Basel, Switzerland]) product transiently, but her anemia did not improve at all.
Initial laboratory test values on admission were as follows: leukocyte count, 4,610 cells/µL; Hb, 5.4 g/dL; platelet count, 113,000 cells/µL; reticulocytes, 0.27%; total iron binding capacity, 220 µg/dL (39.38 µM/L); ferritin, 1,760 µg/L; iron, 201 µg/dL (35.98 µM/L); parathyroid hormone, 23 ng/L; blood urea nitrogen, 83 mg/dL (29.63 mM/L); creatinine, 12.3 mg/dL (1,087.32 µM/L); C-reactive protein, 0.75 mg/dL. Serologic tests for hepatitis viruses, cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, and parvovirus B19 were all negative. Thoracic computed tomographic scans or abdominal sonography showed no evidence of an abnormal mass such as thymoma or lymphoma. Bone marrow examination showed reduced cellularity (0-20%) and severe erythroid hypoplasia, whereas thrombopoiesis was in the low normal range and granulopoiesis was normal, findings consistent with PRCA (Fig. 1).
In June 2003, anti-EPO antibodies were screened by competition enzyme-linked immunoassay (ELISA). The result of ELISA showed 1.9 times higher antibody titer in patients serum compared with in control serum, and ESA treatment was discontinued. The PRCA did not respond to oxymetholone treatment from June 2003 to July 2003. Although we considered immunesuppressive therapies, she refused to receive the treatments. In September 2003, the antibody was also identified by radioimmunoprecipitation assay, their binding capacity was 5.6 IU/mL. In the neutralizing capacity test, her serum showed complete inhibition of colony formation by normal marrow cells when stimulated by 0.5 IU of rEPO-α in an in vitro bioassay.
After then, she remained transfusion-dependent, Espogen was restarted from January 2004, but there was no response. After she was switched to Recormon on SC route, the transfusion requirement decreased and her anemia showed slight improvement. However, her Hb level decreased to 5.3 g/dL again in April 2005. Despite the increased dose of Recormon up to 15,000 IU/week, her Hb level did not recover. In June 2005, the patient was started on another type of ESA therapy, 40 µg/week of Aranesp (darbepoetin-α, Amgen Inc., San Francisco, CA, USA) subcutaneously, but her anemia did not seem to improve. Although darbepoetin-α was replaced with Recormon, there was a big decline in her Hb level again in April 2006. Later, darbepoetin-α (50 mcg/week) was reintroduced, following which her Hb level started to recover slowly and she did not require transfusions from August 2006. Her Hb level increased up to 12.1 g/dL under 20 µg/week of darbepoetin-α treatment in December 2006.
In July 2007, anti-EPO antibodies were still detectable by radioimmunoprecipitation testing, but the concentration was too low for titration. Similarly, biosensor immunoassay (Biacore 3000; GE Healthcare Life-science Corp., UK) detected antibodies against both EPO-α and darbepoetin-α, but their titers were below the quantifiable levels of 80 ng/mL and 100 ng/mL, respectively. The neutralizing capacity was not detected in an in vitro bioassay. Until now, the patient is on subcutaneous darbepoetin-α treatment and the Hb level is maintained at around 9-11 g/dL (Fig. 2).
There is no consensus on the optimal therapy for ESA-induced PRCA. Most often, withdrawal of all kinds of ESAs has been recommended, because there is substantial cross-reactivity among the ESAs, including darbepoetin-α and endogenous EPO (1, 6). Further, immunosuppressive therapy is usually required to lower the anti-EPO antibodies. According to the RADAR Project (3), the recovery rates from PRCA are 2% without immunosuppressive therapy but reach 52% after immunosuppressive therapy and 95% after kidney transplantation. Cyclosporine, corticosteroids, and cyclophosphamide are widely used, but other immune-modulating measures including intravenous immunoglobulins, rituximab (7), and plasma exchange with immunoadsorption (8) can also be tried. Although patients with ESA-associated PRCA are unlikely to respond to treatment with other ESAs, a rechallenge using an alternative ESA has been successful in some cases where the anti-EPO antibody titer declined to below or around its lower detection limit after treatment with immunosuppressants (7, 9-13). In fact, PRCA patients with chronic kidney disease are persistently exposed to the causative antigen even after the removal of exogenous ESAs because a small amount of endogenous EPO is produced continuously in these patients. Therefore, withdrawal of all kinds of ESAs might not be the absolute solution in the treatment of ESA-induced PRCA, and an ESA rechallenge is recommended for patients with ESA-induced PRCA when the anti-EPO antibody titer is low (4).
Most of the patients showing a good response to an ESA rechallenge had experienced a successful response to immunosuppressive therapy before the rechallenge. However, our patient did not show an adequate response to short-term oxymetholone treatment, because her anemia was not corrected and the anti-EPO antibody titer was still high. Instead, the EPO antibody titer seemed to decline spontaneously over time. A similar case has been reported in which darbepoetin-α rescued ESA-induced PRCA successfully without immunosuppressive therapy in spite of the presence of anti-EPO antibodies; interestingly, the anti-EPO antibody titer declined spontaneously, as in our case, when darbepoetin-α treatment was attempted (13). Another case showed darbepoetin-α was rechallenged successfully when anti-EPO antibody titer remained still high; however, several immunesuppressive regimens were successively tried in that case (14).
Different glycosylation patterns of darbepoetin-α might reduce the affinity of anti-EPO antibodies toward darbepoetin-α compared with that toward other ESAs. Differences in ESA formulation might also contribute to the variable immunogenicity of different ESAs. For example, the exposure-adjusted PRCA incidence per 10,000 chronic kidney disease patients was 2.7 with subcutaneous human serum albumin (HSA)-free Eprex, whereas it was 0.2 with subcutaneous HSA-containing Eprex or epoetin-β (15). It is assumed that the relatively lower affinity of anti-EPO antibodies toward darbepoetin-α can overcome the inhibitory effects of these antibodies, especially when their titer declines (13). Similarly, novel ESAs such as Hematide (Affymax Inc., Palo Alto, CA, USA), a pegylated synthetic peptide, can also be administered instead of darbepoietin-α, because anti-EPO and anti-Hematide antibodies did not cross-react with Hematide and EPO, respectively, in an PRCA experimental model (16). Further studies are, however, needed to identify the precise epitopes of various ESAs interacting with EPO receptors in order to support our hypothesis.
In summary, we have reported a rare case of ESA-induced PRCA that improved successfully following the reintroduction of darbepoetin-α without further immunosuppression in the presence of a low level of anti-darbepoetin-α antibodies. This case suggests that darbepoetin-α can be used in a rechallenge to treat ESA-induced PRCA when the anti-EPO antibody titer declines and loses its neutralizing capacity.
Figures and Tables
Fig. 1
Bone marrow biopsy findings. (A) Bone marrow section, The cellularity is 0-20% which is hypocellular for age. Trilineage hematopoiesis is markedly decreased, and the decrease of erythropoiesis is remarkable. Plasma cells, lymphocytes and eosinophils are unremarkable. Foreign cells and granulomata are absent, H&E stained, ×100. (B) A megakaryocyte and myeloid precursor cells are observed, but no erythroid precursor cells are observed, H&E stained, ×1,000.
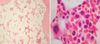
Fig. 2
Clinical course of the patient by time. The gray line is a schematic representation of the patient's hemoglobin (Hb) levels (conversion factor from g/dL to g/L, ×10) from June 2000 to April 2009. One red triangle means transfusion of two units of packed red blood cells. Hemodialysis (HD) was initiated in January 2002. Oxymetholone therapy was given from June 2003 to July 2003. The double-headed arrows below the graph indicate the durations of erythropoiesis-stimulating agent use, represented by the following colors: green arrow, Epokine; blue arrows, Espogen; violet arrows, Recormon; yellow arrows, Aranesp.

References
1. Weber G, Gross J, Kromminga A, Loew HH, Eckardt KU. Allergic skin and systemic reactions in a patient with pure red cell aplasia and anti-erythropoietin antibodies challenged with different epoetins. J Am Soc Nephrol. 2002. 13:2381–2383.

2. Casadevall N, Dupuy E, Molho-Sabatier P, Tobelem G, Varet B, Mayeux P. Autoantibodies against erythropoietin in a patient with pure red-cell aplasia. N Engl J Med. 1996. 334:630–633.

3. Bennett CL, Cournoyer D, Carson KR, Rossert J, Luminari S, Evens AM, Locatelli F, Belknap SM, McKoy JM, Lyons EA, Kim B, Sharma R, Costello S, Toffelmire EB, Wells GA, Messner HA, Yarnold PR, Trifilio SM, Raisch DW, Kuzel TM, Nissenson A, Lim LC, Tallman MS, Casadevall N. Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: a follow-up report from the Research on Adverse Drug Events and Reports (RADAR) Project. Blood. 2005. 106:3343–3347.

4. Macdougall IC, Roche A, Rossert J, Casadevall N, Francois P, Kemeny DM. Re-challenging patients who developed pure red cell aplasia with epoetin: can it be done? Nephrol Dial Transplant. 2004. 19:2901–2905.

5. Yang J, Joo KW, Kim YS, Ahn C, Han JS, Kim S, Lee JS. Two cases of pure red-cell aplasia due to anti-erythropoietin antibodies. J Nephrol. 2005. 18:102–105.
6. Summers S, Holdsworth S, Sharples E. The (re)challenging question of erythropoiesis-stimulating agents inducing pure red cell aplasia. Nephrol Dial Transplant. 2008. 23:3053–3055.

7. Mandreoli M, Finelli C, Lopez A, Ascani S, Vianelli N, Baccarani M, Santoro A. Successful resumption of epoetin alfa after rituximab treatment in a patient with pure red cell aplasia. Am J Kidney Dis. 2004. 44:757–761.

8. Westerlund P, Kurkus J, Segelmark M. Rapid resolution of EPO-induced pure red cell aplasia after a course of immunoadsorption therapy using protein A columns. Am J Kidney Dis. 2005. 45:e97–e99.

9. Pollock C, Johnson DW, Horl WH, Rossert J, Casadevall N, Schellekens H, Delage R, De Francisco A, Macdougall I, Thorpe R, Toffelmire E. Pure red cell aplasia induced by erythropoiesis-stimulating agents. Clin J Am Soc Nephrol. 2008. 3:193–199.

10. Rossert J, Macdougall I, Casadevall N. Antibody-mediated pure red cell aplasia (PRCA) treatment and re-treatment: multiple options. Nephrol Dial Transplant. 2005. 20:Suppl 4. iv23–iv26.

11. Vartia A, Asola MR, Tertti R, Kunelius P, Metsarinne KP. Two haemodialysis patients with epoetin alfa-induced pure red-cell aplasia recovered despite treatment with another epoetin preparation. Nephrol Dial Transplant. 2004. 19:1313–1316.

12. Summers SA, Matijevic A, Almond MK. Successful re-introduction of recombinant human erythropoietin following antibody induced pure red cell aplasia. Nephrol Dial Transplant. 2004. 19:2137–2139.

13. Asari A, Gokal R. Pure red cell aplasia secondary to epoetin alpha responding to Darbepoetin alpha in a patient on peritoneal dialysis. J Am Soc Nephrol. 2004. 15:2204–2207.

14. Viron B, Dupuy CA, Kolta A, Casadevall N. Successful re-challenge with darbepoetin in a patient with rHu-EPO-induced pure red cell aplasia refractory to immunosuppressive drugs. Nephrol Dial Transplant. 2008. 23:2416–2418.

15. Cournoyer D, Toffelmire EB, Wells GA, Barber DL, Barrett BJ, Delage R, Forrest DL, Gagnon RF, Harvey EA, Laneuville P, Patterson BJ, Poon MC, Posen GA, Messner HA. Anti-erythropoietin antibody-mediated pure red cell aplasia after treatment with recombinant erythropoietin products: recommendations for minimization of risk. J Am Soc Nephrol. 2004. 15:2728–2734.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download