Abstract
The aim of this study was to evaluate the effect of early motor balance and coordination training on functional recovery and brain plasticity in an ischemic rat stroke model, compared with simple locomotor exercise. Adult male Sprague-Dawley rats with cortical infarcts were trained under one of four conditions: nontrained control, treadmill training, motor training on the Rota-rod, or both Rota-rod and treadmill training. All types of training were performed from post-operation day 1 to 14. Neurological and behavioral performance was evaluated by Menzies' scale, the prehensile test, and the limb placement test, at post-operation day 1, 7, and 14. Both Rota-rod and treadmill training increased the expression of synaptophysin in subcortical regions of the ischemic hemisphere including the hippocampus, dentate gyrus, and thalamus, but did not affect levels of brain-derived neurotrophic factor or tyrosin kinase receptor B. The Rota-rod training also improved Menzies' scale and limb placement test scores, whereas the simple treadmill training did neither. The control group showed significant change only in Menzies' scale score. This study suggests that early motor balance and coordination training may induce plastic changes in subcortical regions of the ischemic hemisphere after stroke accompanied with the recovery of sensorimotor performance.
Neuroplasticity is an important mechanism of functional recovery after brain injury (1, 2). Neurotrophins have been proposed to play a role in neuronal survival, proliferation, maturation, and outgrowth during development, and to show neuroprotective functions after brain injury (3, 4). Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family and is most abundantly expressed in the central nervous system. The effect of BDNF on neuronal cells is mediated by its high-affinity receptor, tyrosin kinase receptor B (TrkB) (5). Synaptophysin is a pre-synaptic vesicle protein found in all nerve terminals. Synaptophysin measurements have been used to quantify the number of terminals present during neuroanatomical remodeling and neural development (2, 6, 7).
Several authors has described relationships between physical activity and neuroplasticity in animal studies (8-10). These workers showed that voluntary exercise in healthy rats increased the expression of some neurotrophins in the central nervous system including the cerebral cortex, hippocampus and dentate gyrus. It has also been reported that physical activity could enhance neuroplasticity and improve recovery of motor function after brain injury in animal models (11-13), although premature voluntary exercise might compromise neuroplasticity after traumatic brain injury (14), and the intensity of exercise intervention influenced the expression of neurotrophins in a rat stroke model (11).
It has been shown that complex motor skill training, requiring the integration of a variety of inputs and plastic changes across multiple brain structures, had more beneficial effects on outcomes after brain injury than simple repetitive exercise (7, 15, 16). The Rota-rod training, acrobatic training, and tray-reaching task were used as complex motor skill training modes in these studies. The Rota-rod test is an established motor task to evaluate balance and coordination aspects of motor performance in rats (17). Motor balance and coordination training on the Rotarod induced intense synaptophysin immunoreactivity in the thalamus of the ischemic hemisphere (7), and showed better functional outcomes than simple exercise on a treadmill (15), after 14 days of training. Although these results suggest that complex motor skill training is a more favorable exercise protocol than simple exercise for functional recovery and neuroplasticity development after brain injury, the expression of neurotrophins in subcortical regions has not been evaluated precisely and evidence on such expression is still lacking. More studies are required to determine the optimal timing of complex motor skill training after ischemic stroke, to yield optimal recovery outcomes.
The purpose of this study was to investigate the effect of early motor balance and coordination training, using the Rota-rod, on motor function and the expression of neurotrophins, as compared with simple locomotor exercise using a treadmill, in an ischemic rat stroke model. We also employed the simultaneous Rota-rod and treadmill training to investigate the possible synergistic effect of two different types of exercise.
Sixty adult male Sprague-Dawley rats (weighing 250-320 g and aged 6 weeks) were used for the investigation. Throughout the experiments, animals were housed in standard cages; three animals per cage with free access to food and water and a 12:12 hr light/dark cycle. After induction of focal cerebral ischemia, the rats were trained under four different conditions: no exercise, simple locomotor training on a treadmill, motor balance and coordination training on the Rota-rod, and the Rota-rod training with simple treadmill training. On postoperative day 15, rats were sacrificed by rapid decapitation and cerebral hemispheres were immediately dissected for Western blot analysis and immunohistochemistry. The protocols for the care and use of animals were in compliance with international guidelines and were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (IACUC number: 06-144).
To induce focal cerebral ischemia, the intraluminal suture middle cerebral artery occlusion (MCAO) method was used as previously described (18). In brief, after an intraperitoneal injection of 1% (w/v) ketamine (30 mg/kg) and xylazine hydrochloride (4 mg/kg), the left common carotid artery was exposed at its bifurcation through a midline incision of the neck. The proximal portions of the common carotid artery and the external carotid artery were ligated with 5-0 silk suture. Next, a 4.0 monofilament nylon suture, with the tip rounded by heating near a flame, was inserted into the bifurcation site of the common carotid artery. The suture was advanced 16-18 mm into the internal carotid artery from the bifurcation site. Mild resistance indicated that the suture was properly lodged in the anterior cerebral artery and thus blocked blood flow to the middle cerebral artery. For temporary MCAO, reperfusion was obtained by withdrawing the suture gently after 2 hr.
Twenty-four hours after the surgery, ischemic rats were randomly assigned to one of the training conditions. The Rota-rod group exercised on the Rota-rod (FINE-SP-ROTA8080, EGR FINE, Seoul, Korea) at a speed of 35 rpm (8 m/min) for 30 min per day from postoperative day 1 to day 14. If they fell from the Rota-rod during training, they were placed on the rod again (Fig. 1). The treadmill group exercised on a rat treadmill (MJ-262TRT, MJ Ltd., Seoul, Korea) at the same speed (8 m/min) during the same period time. The rat treadmill gave painful electrical shocks to rats that stayed at the rear end of the machine without running. The Rota-rod with treadmill group exercised on the Rota-rod for 15 min and on the treadmill for 15 min per day at the same speed. For conditioning to Rota-rod training, the rats exercised at a speed of 15-25 rpm during the initial 3 to 5 days. The control group was housed in standard cages for an equal duration of time.
Motor function was evaluated by a researcher blind to training conditions using the prehensile test, Menzies' scale, and the limb placement test, at 1, 7, and 14 days postoperatively.
This test was a modified version of the original test (19) which quantitatively evaluated the motor function of ischemic stroke rats. A steel rope, 70 cm long and 5 mm in diameter, was stretched horizontally 60 cm above a table and the forepaws of a rat were placed on the rope and released. The time spent in hanging on the rope was measured until 60 sec had passed.
Menzies' scale is a five-point neurological evaluation scale for the MCAO rat model (20). The scale runs from 0 to 4, and a higher score indicates a more severe neurological deficit.
This was a modified version of a previously described test (21). The test had four tasks assessing the sensorimotor integration of the forelimb and the hind limb by checking responses to tactile and proprioceptive stimulation. Scores from 0 to 10 are obtained, and a higher score indicates a worse sensorimotor performance.
Entire cerebral hemispheres were individually homogenized in 600 µL of 50 mM Tris buffer (pH 7.4, 4℃) containing 1 mM EDTA, 174 µg/mL phenylmethylsulfonyl fluoride, 0.7 µg/mL pepstatin A, 0.5 µg/mL leupeptin, and 2.0 µg/mL aprotinin (iNtRON, Seongnam, Korea). Protein concentrations were determined by the Bradford method (PRO-MEASURETM kit; iNtRON). Homogenates were mixed with aspartate sample buffer (KOMABIOTECH, Seoul, Korea) and boiled for 3 min. Protein separation was performed using 12% (w/v) polyacrylamide with 0.4% (w/v) bis-acrylamide. Proteins were transferred to polyvinylidene fluoride membrane, and blots were probed with anti-BDNF (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-synaptophysin (1:50,000, Santa Cruz Biotechnology). Peroxidase anti-rabbit IgG (1:10,000, Santa Cruz Biotechnology) and anti-mouse IgG (1:10,000, Santa Cruz Biotechnology) were used as secondary antibodies, respectively. Bands were developed on autoradiographic film using a chemiluminescence technique (WEST-ZOL® [Plus], iNtRON). Exposure time was about 10 min for BDNF detection and 30 sec for synaptophysin development. Film signals were scanned using a high-resolution scanner and optical densities of scanned images were measured using Bio-Profile Bio-1D software (Vilber Lourmat; Marne-la-Vallee, France).
Immunohistochemical staining was performed according to the manufacturer's protocol based on the conventional streptavidin-biotin-peroxidase method. Representative sections of 3 mm thickness were rehydrated after deparaffinization with xylene. Antigen retrieval was performed, after which the sections were washed with citrate buffer. Next, sections were immersed in 3% (w/v) H2O2 for 10 min to inhibit endogenous peroxidase activity. After washing three times with phosphate-buffered saline (PBS) over the course of 5 min, sections were incubated with various primary antibodies. The primary antibodies used were anti-BDNF, anti-TrkB, and anti-synaptophysin, all from Santa Cruz Biotechnology, and all used at 1:200. Antigen retrieval was achieved by boiling in a microwave oven after immersion in citrate buffer. The chemical 3,3-diaminobenzidine tetrahydrochloride (DAB) was used as a chromogen. Counterstaining with Meyer's hematoxylin stain followed. As a positive control, known positive brain samples were employed and, for negative controls, primary antibodies were omitted.
For semiquantitative analysis, light microscopic images of the hippocampus (CA1 to CA4 regions), dentate gyrus, and thalamus (×40) were digitized into 2,572×1,904 pixel RGB color images using a high-resolution CCD camera (Axiocam MRc5, Carl Zeiss, Oberkochen, Germany). The images were then converted into 8-bit 256 grey-scale images and the relative optical density (ROD) of each image was measured using an MCID image analysis system (Imaging Research, Ontario, Canada). Each brain region was manually outlined in the digital image and integrated optical densities were measured after subtraction of the background grey level.
One-way ANOVA and repeated-measures ANOVA were performed to compare the results of prehensile time between the different groups and between postoperative days. Menzies' scale data and limb placement test scores were compared by non-parametric tests such as Kruskal Wallis test and Friedman test. If there was a significant difference, Mann-Whitney U test and Wilcoxon signed ranks test were used for further analysis. Evaluation of differences in the RODs of various groups was performed using one-way ANOVA followed by the Tukey post-hoc test. The paired t-test was used to determine side differences in the RODs of brain regions. All statistical analyses were performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Values are shown as means±S.E. The level of significance was set at P<0.05.
A total of 45 rats (10 in the no-exercise control group, 12 in the treadmill training group, 12 in the Rota-rod training group, 11 in the Rota-rod with treadmill training group) with MCAO in the test groups completed the training protocols. The 9 rats that died during experimental procedures and the 6 rats that showed no neurological deficits on postoperative day 1 were excluded. The number of rats that died in each training group was comparable with that in the control group. There were no significant differences in prehensile time, Menzies' scale data, or limb placement test scores between the groups at 1, 7, or 14 days postoperatively. The Rota-rod training group showed significant changes over time in both Menzies' scale and limb placement test scores. The control group and Rota-rod with treadmill group showed improvement only in one of those tests. There was no significant change in the treadmill training group (Fig. 2).
The four groups (n=3 for each group) were analyzed for BDNF and synaptophysin in each cerebral hemisphere using Western blotting. Bands of precursor BDNF (about 30 kDa in size) and mature BDNF (about 14 kDa) were observed. Bands of mature BDNF were quantified by densitometry. The expression of BDNF increased in the Rota-rod training group, especially in ischemic hemispheres, but this was not statistically significant (Fig. 3A). Synaptophysin bands were observed at about 38 kDa. The expression of synaptophysin showed no significant difference between groups (Fig. 3B).
The expression of BDNF, TrkB, and synaptophysin in the four different groups (n=5 in each group) was investigated in the hippocampus, dentate gyrus, and thalamus, by immunohistochemistry. The ROD was calculated as a percentage of signals from each right brain region of the no-exercise control group. There was no significant difference in the expression of BDNF and TrkB between the groups (Fig. 4A, B). A significant increase in synaptophysin immunoreactivity was detected in the hippocampus, dentate gyrus, and thalamus of the ischemic hemisphere of the treadmill training (P<0.05) and the Rota-rod training groups (P<0.01), as compared with the no-exercise control group (Figs. 4C, 5). Compared with the contralateral brain regions, the expression of synaptophysin in the dentate gyrus and thalamus of the ischemic hemisphere increased in the Rota-rod training group (P<0.05).
This study showed that both early motor balance and coordination training on the Rota-rod, and simple treadmill training for 2 weeks, increased synaptophysin immunoreactivity, but not that of BDNF or TrkB, in subcortical regions such as the hippocampus, dentate gyrus, and thalamus of the ischemic hemisphere in an MCAO rat model. The Rota-rod training also improved both motor function and sensorimotor performance of those rats, whereas the simple treadmill training did neither. Rats experiencing simultaneous Rota-rod and treadmill training showed no better outcomes than did animals under the Rota-rod training only.
Synaptophysin is useful for the identification of axonal sprouting and synaptogenesis. In the present study, increased expression of synaptophysin in the subcortical regions of the ischemic hemisphere may represent increased plastic changes in these regions. It has been suggested that post-injury behavioral experience plays an adaptive role in modifying the functional organization of the remaining, intact, cortical tissue (22). Plastic changes have been demonstrated in subcortical regions including the thalamus and brain stem which might make a substantial contribution to representational changes in the cortex (23). Increased synaptogenesis occurring after motor skill training was reported in the cerebral cortex (6) and thalamus (7), in rats recovering from ischemic stroke. Our results showed increased synaptogenesis in the subcortical regions in animals receiving both motor balance and coordination training, and simple treadmill training. However, synaptophysin expression was significantly higher in ischemic hemispheres than contralateral hemispheres only in the motor balance and coordination training group. It is possible that motor balance and coordination training enhanced plastic changes of subcortical regions which had lost connections from the ipsilateral cerebral cortex after ischemic brain damage.
Although immunohistochemical staining showed different synaptophysin immunoreactivity between groups, these differences were not found in Western blot analysis. We analyzed only a small number of rat brains to examine BDNF and synaptophysin expression in the whole cerebral hemisphere. In previous studies, an enriched environment (24), or motor balance and coordination training (7), influenced the expression of BDNF or synaptophysin in some brain regions, but not in other regions. Western blot analysis of the whole cerebral hemisphere might be inadequate to evaluate changes in defined brain regions. Another possibility is varying tissue loss between brains of different training groups. Light-microscopic images showed a wide variability of tissue loss induced by MCAO in the cerebral cortices of ischemic hemispheres. The degree of tissue loss would influence protein quantification.
BDNF has been suggested to have neuroprotective functions after brain injury (25). Previous studies have demonstrated that physical activity and learning induces BDNF mRNA and protein in the hippocampus of rat brain (8, 10). However, the training groups in our study did not show any significant difference in the expression of BDNF when compared with the control group. Klintsova et al. (13) have reported that both complex motor learning and moderately paced running exercises affected the expression of BDNF in the motor cortex and cerebellum, although the expression of TrkB differed with training conditions. These authors suggested that the involvement of BDNF and its receptor was not exclusively coupled to the synaptic plasticity accompanying complex motor skill learning. Moreover, there might be differences between healthy rats and ischemic stroke rats. Ischemic stroke induces changes in BDNF brain expression and physical activity modulates such expression. Zhao et al. (26) reported that postischemic BDNF gene expression was lower in most parts of the hippocampus of rats housed in an enriched environment rather than in a standard cage. These authors suggested that early postischemic dampening of neuronal activity might be beneficial, or that there is no causality between BDNF levels and functional outcome. Vaynman et al. (27) reported that exercise-induced increases in synapsin I and synaptophysin in the hippocampus were abrogated by blocking of interaction between BDNF and the BDNF receptor TrkB. Our results showed increased synaptophysin immunoreactivity in the hippocampus and dentate gyrus of ischemic hemispheres of both training groups, although BDNF immunoreactivity did not change. The interval between the end of training and the sacrifice of rats may be an important factor. Synaptophysin is a pre-synaptic vesicle protein, the amount of which reflects the number of active terminals, whereas BDNF is a neurotrophic factor. Rats were sacrificed 1 day after the last training session, and this might have influenced the immunohistochemical results. Further investigations are required to understand the effect of exercise or motor skill training on the expression of BDNF, an important modulator of synaptogenesis in the hippocampus, in ischemic stroke rats.
Several studies have reported that treadmill exercise or complex motor skill training improved functional outcomes in rats after brain injury (12, 15, 16, 28). Our results showed significant improvement of function in the rats trained on the Rota-rod, but not in those trained on the treadmill. In previous studies using the Rota-rod for complex motor skill training, the training protocol began 5 days after surgery (7, 15). The effect of early physical activity on functional outcomes after brain injury is controversial (28-30). Yang et al. (28) indicated that early treadmill training, commenced 24 hr after MCAO, had significant effects in reducing brain infarct volume and in improving neurological function, when compared with spontaneous recovery. The same effects were not shown when late training started 1 week after MCAO. We designed our training protocols to commence 24 hr after MCAO in the present study to investigate the effect of early motor balance and coordination training. This early training protocol improved sensorimotor integration as well as motor function in the Rota-rod training group. In the simple locomotor training group, the speed of the rat treadmill was adjusted to 8 m/min, the same as that of the Rota-rod. This is relatively low-intensity exercise compared with the speed of the treadmill (20 m/min) used in previous studies which investigated animals recovering from stroke (12, 28). Low-intensity treadmill training might be insufficient to improve neurological and behavioral outcomes although such training induced the expression of brain synaptophysin.
Unlike other training groups, there was no significant difference in synaptophysin expression of subcortical regions between the Rota-rod with treadmill training group and the control group. This simultaneous training protocol was employed to investigate the possible synergistic effect of two different types of exercise. However, the Rota-rod with treadmill training seemed to be inappropriate for an exercise protocol in the present study. It has been indicated that exercise induced synaptic plasticity in the hippocampus and thalamus were associated with learning capability (9) and volitional motor learning in rats (7). Fifteen minutes might be too short to accustom ischemic stroke rats to each type of exercise, particularly the Rota-rod training. As a result, this training protocol was inadequate to induce motor learning with synaptic changes in subcortical regions. In addition, the training protocol with two different type of exercise might be stressful to rats in early period after ischemic stroke. Under a training condition, exercise could disrupt the upregulation of plasticity-related proteins in the early period after brain injury (14). The inconsistent results from the Rota-rod with treadmill training group are supposed to arise from these possible drawbacks.
There were some limitations in this study. We did not assess the infarct size or cell loss of the brain tissue which might be useful to evaluate the possible degenerative effect of early intense training. It might be also helpful in interpreting the result of Western blot analysis of the whole cerebral hemisphere. In addition, a sham operation was needed to evaluate the effect of ischemia on behavior or protein expression. Our results were limited to show differences between the training groups and no-exercise control group. If a sham operation group was included in our study, the effect of training could be indicated separately from the effect of ischemia.
In the present article, we have shown that both motor balance and coordination training, and low intensity treadmill training, both of which started 24 hr after surgery, induced synaptogenesis in subcortical regions of the ischemic hemisphere including the hippocampus, dentate gyrus, and thalamus, in an MCAO rat model. However, only motor balance and coordination training showed beneficial effects on sensorimotor performance. These results suggest that early complex motor training may induce plastic changes in subcortical regions of the ischemic hemisphere after stroke accompanied with the recovery of sensorimotor performance. For better functional recovery after stroke, we need to consider the optimal timing and intensity of training, as well as the type of training to be offered.
Figures and Tables
 | Fig. 1A photograph of Rota-rod training by the rats. The rats were placed on the Rota-rod cylinder which rotated at a speed of 35 rpm. If they fell, they were placed on the rod again. |
 | Fig. 2Neurological and behavioral test scores. (A) There is no significant difference in prehensile time. (B) The Rota-rod and control groups show significant difference over time in Menzies' sclae. (C) The Rota-rod and Rota-rod with treadmill groups show significant difference over time in limb placement test score.
*P<0.05 by Friedman test and Wilcoxon signed ranks test.
|
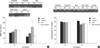 | Fig. 3Western blot analysis. (A) Relative levels of BDNF in entire cerebral hemispheres show no significant between-group difference. A sample blot is shown; mature BDNF (about 14 kDa in size) is seen. (B) Relative levels of synaptophysin in entire cerebral hemispheres show no significant difference between groups. A sample blot is shown; synaptophysin (about 38 kDa in size) is noted. |
 | Fig. 4Immunohistochemical analysis. The RODs of BDNF, TrkB, and synaptophysin in each brain region are represented as percentages of each contralateral brain region of the control group. There is no significant between-group difference in the expression of (A) BDNF, or (B) TrkB by one-way ANOVA. (C) Significant differences in synaptophysin immunoreactivity are detected in the treadmill and the Rota-rod training groups as compared with the control group. Hippo, hippocampus; Den, dentate gyrus; Thal, thalamus.
*P<0.05, †P<0.01 vs no exercise control group (one-way ANOVA and Tukey's post-hoc test); ‡P<0.05 vs contralateral brain regions (paired t-test).
|
 | Fig. 5Synaptophysin expression by immunohistochemical staining. High-power views (×400) of the hippocampal dentate gyrus (A-D, I, J) and thalamus (E-H) show synaptophysin immunoreactivity in brown color. No definite differences are observed in the granule cell layer and subgranular zone of the contralateral hemisphere between the no exercise control (A) and Rota-rod training (B) groups. In the ischemic hemisphere, synaptophysin expression increases in the Rota-rod training group (D) compared to the control group (C). Synaptophysin immunoreactivity is higher in the thalamus of the Rota-rod training group (F, H) than in that of the control group (E, G), in particular, in the ischemic hemisphere (H). The images of the dentate hilus of the Rota-rod group (I, J) also show synaptophysin expression, particularly in the ischemic side (J). |
References
1. Johansson BB, Grabowski M. Functional recovery after brain infarction: plasticity and neural transplantation. Brain Pathol. 1994. 4:85–95.

2. Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995. 26:2135–2144.

4. Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000. 14:2919–2937.

5. Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994. 17:490–496.

6. Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996. 16:4529–4535.

7. Ding Y, Li J, Clark J, Diaz FG, Rafols JA. Synaptic plasticity in thalamic nuclei enhanced by motor skill training in rat with transient middle cerebral artery occlusion. Neurol Res. 2003. 25:189–194.

8. Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996. 726:49–56.

9. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999. 96:13427–13431.

10. Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004. 76:356–362.

11. Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005. 136:991–1001.

12. Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 2005. 1052:16–21.

13. Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004. 1028:92–104.

14. Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004. 1016:154–162.

15. Ding Y, Li J, Lai Q, Rafols JA, Luan X, Clark J, Diaz FG. Motor balance and coordination training enhances functional outcome in rat with transient middle cerebral artery occlusion. Neuroscience. 2004. 123:667–674.

16. Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008. 22:250–261.

17. Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997. 28:2060–2065. discussion 6.

18. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.

19. Combs DJ, D'Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke. 1987. 18:503–511.

20. Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992. 31:100–106.
21. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003. 34:2258–2263.

22. Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001. 24:1000–1019.

23. Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998. 282:1121–1125.

24. Zhao LR, Risedal A, Wojcik A, Hejzlar J, Johansson BB, Kokaia Z. Enriched environment influences brain-derived neurotrophic factor levels in rat forebrain after focal stroke. Neurosci Lett. 2001. 305:169–172.

25. Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, Siesjo BK, Lindvall O. Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol. 1995. 136:73–88.

26. Zhao LR, Mattsson B, Johansson BB. Environmental influence on brain-derived neurotrophic factor messenger RNA expression after middle cerebral artery occlusion in spontaneously hypertensive rats. Neuroscience. 2000. 97:177–184.

27. Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006. 1070:124–130.

28. Yang YR, Wang RY, Wang PS. Early and late treadmill training after focal brain ischemia in rats. Neurosci Lett. 2003. 339:91–94.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download