Abstract
Early and accurate differentiation between infectious and non-infectious fever is vitally important in the intensive care unit (ICU). In the present study, patients admitted to the medical ICU were screened daily from August 2008 to February 2009. Within 24 hr after the development of fever (>38.3℃), serum was collected for the measurement of the procalcitonin (PCT) and high mobility group B 1 levels. Simplified Acute Physiology Score (SAPS) II and Acute Physiology And Chronic Health Evaluation (APACHE) III scores were also analyzed. Sixty-three patients developed fever among 448 consecutive patients (14.1%). Fever was caused by either infectious (84.1%) or non-infectious processes (15.9%). Patients with fever due to infectious causes showed higher values of serum PCT (7.8±10.2 vs 0.5±0.2 ng/mL, P=0.026), SAPS II (12.0±3.8 vs 7.6±2.7, P=0.006), and APACHE III (48±20 vs 28.7±13.3, P=0.039) than those with non-infectious fever. In receiver operating characteristic curve analysis, the area under the curve was 0.726 (95% CI; 0.587-0.865) for PCT, 0.759 (95% CI; 0.597-0.922) for SAPS II, and 0.715 (95% CI; 0.550-0.880) for APACHE III. Serum PCT, SAPS II, and APACHE III are useful in the differentiation between infectious and non-infectious fever in the ICU.
Fever, defined as an increase in body temperature above 38.3℃ (100.4°F), occurs in approximately one-third of all medical patients during their hospital stay (1). Intensive care unit (ICU) patients frequently develop fevers which can originate from infectious or non-infectious causes (2-4). The main causes of non-infectious fever include myocardial infarction, pulmonary embolism, deep vein thrombosis, cerebral infarction, hemorrhage, atelectasis, pancreatitis, acalculus cholecystitis, drug fever, and postoperative fever. On the other hand, major causes of infectious fever in the ICU include ventilator-associated pneumonia, sinusitis, catheter-related infections, nosocomial diarrhea, and wound infections (5).
Fever has multiple clinical effects: increased energy expenditure, myocardial, and respiratory demands, as well as discomfort and worse central nervous system (CNS) injury. On the other hand, fever is regarded as a beneficial host immune response to infection (6). Fever frequently leads to a series of diagnostic work-ups which significantly increase medical costs and expose the patient to the risk of invasive procedures. Empirical treatment of fever, which frequently follows diagnostic work-ups, may result in the inappropriate use of antibiotics and an increase in antibiotic- resistant pathogens. As such, early and accurate differentiation between infectious and non-infectious fever is very important.
Several biologic markers have been tested for their ability to discriminate between infectious and noninfectious fever, including serum procalcitonin (PCT), C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) (7). However, none of these markers have been proven to have enough power to be useful in clinical practice.
The high mobility group box-1 (HMGB1) protein is a 30 kDa nonhistone nuclear DNA binding protein that has been shown to have an extracellular role in inflammation, cell differentiation, adherence, and motility (8). HMGB1 has also been proposed as one of the mediators of sepsis (9, 10). Therefore, HMGB1 may be useful as a differential marker for the evaluation of fever in ICU patients (11). However, serum HMGB1 has not yet been evaluated for this purpose.
Febrile patients by infectious cause of fever have more chance to develop organ dysfunction and subsequent high score in acute physiology and chronic health evaluation (APACHE) III system and are associated with increased mortality (12). However, APACHE III score and other disease severity score, such as simplified acute physiology score (SAPS) II have not specifically evaluated for the purpose of discrimination between infectious and non-infectious fever in the ICU.
In the present study, we evaluated the value of serum PCT, HMGB1, SAPS II, and APACHE III scores in the differentiation between infectious and non-infectious fever in ICU patients.
A prospective cohort study was conducted at the 21-bed medical intensive care unit (MICU) of Chung-Ang University Hospital, Seoul, Korea, from August 2008 to February 2009. This study was approved by the IRB of Chung-Ang University Hospital and the approval number was C2008015(118). Informed consent was obtained from the family members of the patients.
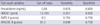
During the 7-month period (August 2008 to February 2009), 448 patients were consecutively admitted to the MICU. Fever developed in 63 of these patients (14.1%). Thirty-five patients were men and 28 were women, and the mean age was 65.2±12.4 yr. Fever developed within three days of admission in 46 of the 63 patients. The mean duration of ICU stay was 25.8±20.2 days, and the mean duration of fever was 3.0±2.4 days. The SAPS II and APACHE III scores were 11.2±3.8 and 45.3±19.5, respectively (Table 1).
Body temperature was measured using the axillary method with an electronic thermometer (Thermoval classic, PAUL HARTMANN AG, German). We defined ICU fever as a body temperature above 38.3℃ that developed 48 hr after ICU admission. Within 24 hr after the development of fever, serum samples were collected and stored at -70℃ and serum PCT and HMGB1 levels were measured later with duplication. A fluorescent enzyme immunoassay (FEIA) (VIDAS BRAHMS PCT, France) was used to measure PCT levels. Serum HMGB1 levels were evaluated with an enzyme-linked immunosorbent assay (HMGB1 ELISA kit, Shino-Test Co, Japan). Clinical parameters, including demographic data, underlying diseases, duration of ICU stay, causes of fever, SAPS II, APACHE III scores, and mortality rate were also analyzed.
Pneumonia was defined as a constellation of symptoms and signs in combination with at least a newly developed infiltrate upon chest radiography. Urinary tract infection (UTI) was defined as pathogenic microorganisms detected in the urine at more than 100,000 organisms/mL (13). Sepsis was defined as systemic inflammatory response syndrome (SIRS) with a proven or suspected microbial etiology (14). Phlebitis was defined as the clinical findings of pain, tenderness, induration, and/or erythema in a superficial vein due to inflammation, infection, and/or thrombosis (15). Post-operative fever was defined as non-infectious fever occurring within the first 4 post-operative days, based on a modification of the description by Clarke et al. (16). Drug fever was defined as a disorder characterized by fever coinciding with the administration of a drug and disappearing after the discontinuation of the drug, when no other cause for the fever was evident after a careful physical examination and laboratory investigation. Fevers were labeled as unknown cause when routine medical and laboratory examination did not reveal the origin of fever.
Most fevers were due to infectious causes (53 cases, 84.1%). Pneumonia (27 cases, 42.9%) was the most common cause of infectious fever. Other causes of infectious fever were sepsis, postoperative wound infection, and urinary tract infection. Causes of non-infectious fever (10 cases, 15.9%) included drug fever, gastrointestinal bleeding, and postoperative fever (Table 2).
Statistical analyses were performed using the Statistical Package for the Social Sciences version 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were compared using the Student t test for normally distributed variables and the Mann-Whitney U test was used for non-normally distributed variables. P values of less than 0.05 were considered significant. The ability of each parameter to discriminate between infectious and non-infectious fever was investigated using a receiver operating characteristic (ROC) analysis.
There were no significant differences in age (P=0.19), gender (P=0.76), serum HMGB1 (P=0.52), and mortality rate (P=0.33) between patients with infectious and non-infectious fever (Table 1). Febrile patients with infectious causes had higher levels of serum PCT than those with non-infectious causes (7.8±10.2 vs 0.5±0.2 ng/mL, P=0.026). In addition, infectious fever was associated with higher SAPS II and APACHE III scores (12±3.8 vs 7.6±2.7, P=0.006; 48±20 vs 28.7±13.3, P=0.039) (Table 1).
The Receiver Operating Characteristic (ROC) curve analysis revealed that the diagnostic performance of PCT and the SAPS II and APACHE III scores for infectious and non-infectious fever was in the good range, and the area under the curve (AUC) was 0.726 (95% CI; 0.587-0.865) for PCT, 0.759 (95% CI; 0.597-0.922) for the SAPS II score, and 0.715 (95% CI; 0.550-0.880) for the APACHE III score (Fig. 1). The optimum cutoff value for distinguishing between infectious and non-infectious fever was 0.68 ng/mL for PCT (sensitivity, 67.6%; specificity, 80.0%), 8.5 points for the SAPS II score (sensitivity, 78.4%; specificity, 70.0%), and 31.5 points for the APACHE III score (sensitivity, 64.9%; specificity, 70.0%) (Table 3), respectively. The combination of PCT, SAPS II and APACHE III scores increased the AUC and diagnostic accuracy (Fig. 2). When we set the cut off value of PCT to 0.68 ng/mL, SAPS II to 8.5 points, and APACHE III to 31.5 points, the combination of all three parameters showed 100% of sensitivity, 25% of specificity, 43% of positive predictive value, and 100% of negative predictive value for the differentiation between infectious and non-infectious fever.
In the present study, we evaluated biologic markers, including serum PCT and HMGB1, and disease severity scores, such as SAPS II and APACHE III, in the differentiation of infectious and non-infectious fever in the ICU. In accordance with the guidelines of the Society of Critical Care Medicine and the Infectious Disease Society of America, we defined an ICU fever as a temperature equal to or above 38.3℃ in patients who stayed in the ICU for at least 48 hr (3).
In the present study, fever developed in 63 patients among 448 consecutive patients admitted to the ICU (14.1%), which was far less as compared to other report (2). The low prevalence of ICU fever in our study might be explained by the fact that we evaluated patients admitted to the medical ICU and excluded patients in the surgical ICU, where fever can be due to many causes, such as wound infection, transfusion reaction, and postoperative fever. Also, our ICU contains a coronary care unit (CCU), and these patients usually have a low risk for infectious disease. In addition, we used the axillary method to check body temperature, and this method usually results in lower temperature compared to other methods (17, 18).
ICU patients frequently have multiple infectious and noninfectious causes of fever, necessitating a systematic and comprehensive diagnostic approach. The most common infection reported in ICU patients is pneumonia, followed by sinusitis, blood stream infection, and catheter-related infection (2, 4, 19). As was reported in our previous study, infectious causes of fever (84.1%) predominated, and pneumonia was the most common cause of infection (42.9%) in the present study (20). While infections are important causes of fever in the ICU, many noninfectious inflammatory conditions result in tissue injury, inflammation, and a febrile reaction. Noninfectious disorders that should be considered in ICU patients are post-operative fever, transfusion reaction, drug fever, and cerebral infarction (15). In the present study, causes of non-infectious fever (10 cases, 15.9%) were drug fever, gastrointestinal bleeding, and postoperative fever (Table 2).
It is well known that early recognition, together with prompt and appropriate treatment of infections, can significantly reduce mortality in critically ill patients (2). The dilemma with ICU fever is to exclude noninfectious causes as soon as possible and then to locate the site of infection and determine the likely pathogens. To date, no single clinical or biological indicator has gained unanimous acceptance in the differentiation between infectious and noninfectious fever in the ICU.
CRP and PCT have been used as objective markers of bacterial infection. Although CRP is a more sensitive marker of sepsis than either body temperature or white blood cell count, it lacks specificity (21). PCT, a propeptide of calcitonin, is found to be an earlier marker of septic shock than CRP and it correlates more closely to severity of disease (22). Pleural effusion PCT was higher in bacterial pneumonia than in non-bacterial infection (23). However, there is a debate for the utility of PCT in distinguishing infection from other causes of systemic inflammatory response syndrome (SIRS) in older patients (24). In the present study, we were able to confirm that serum PCT levels were significantly high in patients with infectious fever (Table 1). Manufacturer's instruction recommends interpreting PCT results with the five categories indicating healthy condition, local infection, sepsis, severe sepsis, and septic shock. However, in the present study, the mean value of PCT in 9 patients with sepsis (6.9 ng/mL) was not different from that of other patients with local infection (7.8 ng/mL).
We also evaluated serum HMGB1 for this purpose because there is abundant evidence supporting the role of HMGB1 as an inflammatory mediator, especially in sepsis (9, 10). To our disappointment, serum HMGB1 proved not to be useful as a tool in the differentiation between infectious and non-infectious fever (Table 1). In the present study, disease severity scores, including the SAPS II and APACHE III scores, were different between infectious and noninfectious fever (Table 3) (Fig. 1). The overlap between the two groups was too large to be used in the differentiation of infectious and non-infectious fever. However, combination of each parameter and especially combination of all three parameters, PCT, SAPS II, and APACHE III score increased AUC and diagnostic accuracy for the differentiation (Fig. 2). Because there is no single clinical or biological indicator that gained unequivocal acceptance in the differentiation between infectious and noninfectious fever in the ICU, the combined analysis of all three parameters might be a practical alternative for this purpose.
Our study has a few limitations. First, we performed the study at only one hospital and evaluated only patients admitted to the medical ICU. We were able to identify candidate parameters for the differentiation of infectious and noninfectious fever (serum PCT, SAPS II, and APACHE III scores). However, there is a limitation in the diagnostic accuracy of three parameters. Despite these limitations, our study may be the rare, prospective study in Korean population to evaluate parameters for the differentiation between infectious and noninfectious fever in the ICU.
In conclusion, serum PCT levels, SAPS II and APACHE III scores demonstrated significant differences between patients with infectious and non-infectious fever and combined analysis of all three parameters increased diagnostic accuracy.
Figures and Tables
Fig. 1
ROC curve for the prediction of infectious and non-infectious fever. HMGB1, high mobility group B 1; SAPS, simplified acute physiology score; APACHE, acute physiology and chronic health evaluation III. The area under the curve was 0.726 (95% CI; 0.587-0.865) for PCT, 0.759 (95% CI; 0.597-0.922) for the SAPS II score, and 0.715 (95% CI; 0.550-0.880) for the APACHE III score.

Fig. 2
Receiver operating characteristic (ROC) curve for the prediction of infectious and non-infectious fever with the combination of PCT, SAPS II and APACHE III scores. ProSAPS, combination of PCT and SAPS II score; proAPA, combination of PCT and APACHE III score; SAPSAPA, combination of SAPS II score and APACHE III score; proSAPSAPA, combination of PCT, SAPS II score, and APACHE III score. The area under the curve was 0.849 (95% CI; 0.746-0.952) for PCT+SAPS II, 0.830 (95% CI; 0.710-0.950) for PCT+APACHE III, 0.862 (95% CI; 0.762-0.962) for SAPS II+APACHE III, and 0.917 (95% CI; 0.848-0.986) for PCT+SAPS II+APACHE III.

References
1. Ryan M, Levy MM. Clinical review: fever in intensive care unit patients. Crit Care. 2003. 7:221–225.
2. Circiumaru B, Baldock G, Cohen J. A prospective study of fever in the intensive care unit. Intensive Care Med. 1999. 25:668–673.

3. O'Grady NP, Barie PS, Bartlett J, Bleck T, Garvey G, Jacobi J, Linden P, Maki DG, Nam M, Pasculle W, Pasquale MD, Tribett DL, Masur H. Task Force of the American College of Critical Care Medicine of the Society of Critical Care Medicine in collaboration with the Infectious Disease Society of America. Practice parameters for evaluating new fever in critically ill adult patients. Crit Care Med. 1998. 26:392–408.
4. Meduri GU, Mauldin GL, Wunderink RG, Leeper KV Jr, Jones CB, Tolley E, Mayhall G. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994. 106:221–235.

5. Dimopoulos G, Falagas ME. Approach to the febrile patient in the ICU. Infect Dis Clin North Am. 2009. 23:471–484.

7. Fraunberger P, Wang Y, Holler E, Parhofer KG, Nagel D, Walli AK, Seidel D. Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006. 26:10–12.

8. Kalyan S, Chow AW. Staphylococcal toxic shock syndrome toxin-1 induces the translocation and secretion of high mobility group-1 protein from both activated T cells and monocytes. Mediators Inflamm. 2008. 2008:512196.

9. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999. 285:248–251.

10. Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004. 170:1310–1316.

11. Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002. 18:231–236.

12. Barie PS, Hydo LJ, Eachempati SR. Causes and consequences of fever complicating critical surgical illness. Surg Infect (Larchmt). 2004. 5:145–159.

13. Schaberg DR, Haley RW, Highsmith AK, Anderson RL, McGowan JE Jr. Nosocomial bacteriuria: a prospective study of case clustering and anti-microbial resistance. Ann Intern Med. 1980. 93:420–424.

15. Soifer NE, Borzak S, Edlin BR, Weinstein RA. Prevention of peripheral venous catheter complications with an intravenous therapy team: a randomized controlled trial. Arch Intern Med. 1998. 158:473–477.
16. Clarke DE, Kimelman J, Raffin TA. The evaluation of fever in the intensive care unit. Chest. 1991. 100:213–220.

17. Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992. 268:1578–1580.

18. Schmitz T, Bair N, Falk M, Levine C. A comparison of five methods of temperature measurement in febrile intensive care patients. Am J Crit Care. 1995. 4:286–292.

19. Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M. EPIC International Advisory Committee. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA. 1995. 274:639–644.
20. Jeon EJ, Lee HM, Cho SG, Kang HK, Kwak HW, Song JH, Jung JW, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. Causes of fever in the ICU - a prospective, cohort study. Korean J Crit Care Med. 2008. 23:13–17.

21. Povoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragao A, Sabino H. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998. 24:1052–1056.
22. Claeys R, Vinken S, Spapen H, ver Elst K, Decochez K, Huyghens L, Gorus FK. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med. 2002. 30:757–762.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download