Abstract
Oxidative stress induced by chronic hyperglycemia in type 2 diabetes plays a crucial role in progressive loss of β-cell mass through β-cell apoptosis. Glucagon like peptide-1 (GLP-1) has effects on preservation of β-cell mass and its insulin secretory function. GLP-1 possibly increases islet cell mass through stimulated proliferation from β-cell and differentiation to β-cell from progenitor cells. Also, it probably has an antiapoptotic effect on β-cell, but detailed mechanisms are not proven. Therefore, we examined the protective mechanism of GLP-1 in β-cell after induction of oxidative stress. The cell apoptosis decreased to ~50% when cells were treated with 100 µM H2O2 for up to 2 hr. After pretreatment of Ex-4, GLP-1 receptor agonist, flow cytometric analysis shows 41.7% reduction of β-cell apoptosis. This data suggested that pretreatment of Ex-4 protect from oxidative stress-induced apoptosis. Also, Ex-4 treatment decreased GSK3β activation, JNK phosphorylation and caspase-9, -3 activation and recovered the expression of insulin2 mRNA in β-cell lines and secretion of insulin in human islet. These results suggest that Ex-4 may protect β-cell apoptosis by blocking the JNK and GSK3β mediated apoptotic pathway.
Diabetes mellitus is a metabolic disease caused by impairment of pancreatic β-cell insulin secretion and increase of peripheral insulin resistance. In diabetes, hyperglycemia developed by progressive failure of pancreatic β-cells. Reduced β-cell mass cannot secrete sufficient amounts of insulin to compensate for the peripheral insulin resistance (1, 2). Sustained and prolonged hyperglycemia triggers the development the late micro- and macro- complications of diabetes (3, 4), also progressive deterioration of β-cell mass and insulin synthesis (5, 6). Oxidative stress and accelerated production of reactive oxygen species (ROS) induced by chronic hyperglycemia plays a key role in diabetic progression and further β-cell loss (5, 7, 8). Although β-cell and tissue damage caused by hyperglycemia-induced oxidative stress has been studied considerably in recent years, exact mechanism and effective therapeutic strategies are still not proven.
Recent evidence has supported the importance of oxidative stress on antiapoptotic pathway (9, 10). The c-Jun-N-terminal kinase (JNK), also known as stress-activated protein kinase (SAPK), is activated by oxidative stress (11). Also, signaling through PI3-kinase and Akt are well-established activators of survival in numerous cell types, and overexpression of Akt specifically in pancreatic islet β-cells resulted in marked expansion of cell number and size (12, 13). Inhibition of Glycogen synthase kinase 3β (GSK3β), the first substrate phosphorylated by Akt (14), by growth factor may be an important mechanism to promote β-cell survival and inhibit apoptosis (15, 16).
Glucagon like peptide-1 (GLP-1), incretin hormone secreted from intestinal L cell, stimulates glucose-dependent insulin secretion in β-cell (17-19). It has trophic factor-like properties, acting to stimulate β-cell growth and differentiation by interaction with GLP-1 receptor (GLP-1R), a member of the Gs-protein-coupled receptor superfamily (17-19). Also, GLP-1 may have an antiapoptotic effect on β-cell (20), but detailed mechanisms are not proven.
Therefore, we examined the protective mechanism of Ex-4 (GLP-1R agonist) in β-cell line and human islet by blocking the GSK3β and JNK mediated apoptotic pathway in oxidative damage.
HIT-T15 cells, a hamster-derived insulin-secreting cell line, were grown in RPMI1640 medium supplemented with 10% heat-inactivated FBS, penicillin (100 U/mL), streptomycin (100 µg/mL), 2 g/L NaHCO3 and glucose was adjusted to 11.1 mM. The cell culture was maintained at 37℃ in a humidified atmosphere containing 5% CO2. Before experiments cells, at 80% confluence, were starved for 12 hr in 0.5% FBS-containing RPMI1640 and then incubated with the H2O2 (100 µM) (Acros organics, Geel, Belgium) for the indicated time. HIT-T15 cells and human islet was pretreated with H89 (10 µM) (Calbiochem, San Diego, CA, USA) or SP600125 (20 µM) (Calbiochem, San Diego, CA, USA). After 30 min, Ex-4 (25 nM) (Bachem Bioscience, King of Prussia, PA, USA), NAC (5 mM) (Sigma, St. Louis, MO, USA) or forskolin (20 µM) (Calbiochem, San Diego, CA, USA) were added to cells for 1 h before treatment of H2O2.
We obtained human islets from Kwang-Won Kim, M.D. in Samsung Medical Center (SMC) in Seoul, Korea. According to the report from SMC, human pancreases were procured from braindead donors after informed consent had been obtained from the donors' relatives at the Samsung Medical Center. Islets were isolated from the whole pancreas using a mechanical and enzymatic dissociation process that has been previously described (21). Briefly, the ducts were perfused with a collagen digest enzyme (Liberase human islet, Roche, Indianapolis, IN, USA). The islets were then separated and purified with the use of continuous gradients of Ficoll-diatrizoic acid (Seromed-Biochrom KG, Berlin, Germany). The islets were counted and scored for size on samples taken from the preparations. After isolation, the islets were cultured for 48 hr for assay and experiment procedures. Then, extracted proteins for western blot analysis and collected media for insulin assay was done.
Cell viability was measured with blue formazan that was metabolized from 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (Sigma, St. Louis, MO, USA) by mitochondrial dehydrogenase, which are active only in live cells. Briefly, HIT-T15 cells were seeded in 24-well plates (1.0×105 cells/mL) and incubated in RPMI 1640 medium for 24 hr. Then, the medium was changed to 0.5% FBS-containing RPMI1640 and cells were incubation for 12 hr. After being incubated with the H2O2 (100 µM) for the indicated time, MTT reagent (5 mg/mL) was added to each of the wells, and the plate was incubated for an additional 4 hr at 37℃. The media were then removed, and the intracellular formazan product was dissolved in 250 µL of DMSO. The absorbency of each well was then measured at 540 nm (ref. 650 nm) using the microplate reader (Molecualr Devices Emax, Sunnyvale, CA, USA). OD values from untreated control cells were designated 100% as a standard.
Apoptosis was investigated by staining the cells with Hoechst 33342 (Sigma, St. Louis, MO, USA). HIT-T15 cells were washed twice with PBS and then fixed in PBS containing 10% formaldehyde for 4 hr at room temperature. Fixed cells were washed with PBS and stained with Hoechst 33342 for 30 min at room temperature. Cells were evaluated under a fluorescence microscope (OlympusBX51; Olympus Corp., Tokyo, Japan) for nuclei showing typical apoptotic features such as chromatin condensation and fragmentation. Photographs were taken at a magnification of ×400.
Surface exposure of phosphatidyl serine in apoptotic cells was quantitatively detected using Annexin V-FITC and PI apoptosis detection kit (Beckton Dickinson Bioscience, San Jose, CA, USA). Briefly, cells were seeded into 6-well plates (1.0×106 cells/mL) and incubated for 24 hr. Then, the medium was changed to 0.5% FBS-containing RPMI1640 and cells were starved for 12 hr. After treatment with the H2O2 for varying times, the cells were harvested and washed twice with ice-cold PBS. After 5 min of centrifuging at 5,000 rpm, Annexin V-FITC and PI double-staining were performed according to manufacturer's instruction. Cell apoptosis was analyzed on a FACSscan flow cytometry (Beckton Dickinson, San Jose, CA, USA). Annexin V-FITC-positive, PI-negative cells were scored as apoptotic. Double-stained cells were considered either as necrotic or as late apoptotic (22).
Caspase-3, -8 and -9 activities were determined by a colorimetric assay using kits from R&D System (Wiesbaden-Nordenstadt, Germany), according to the manufacturer's protocol. Briefly, cells were lysed in the supplied lysis buffer and were incubated on ice for 10 min. At the end of the incubation, cell lysates were centrifuged at 10,000×g for 10 min at 4℃ to precipitate cellular debris. The supernatants were collected and incubated with the supplied reaction buffer containing dithiothreitol and DEVD-pNA (specific for caspase-3) or IETD-pNA (specific for caspase-8) and LEHD-pNA (specific for caspase-9) as substrates at 37℃. The reaction was measured by changes in absorbance at 405 nm using an ELISA reader (Molecualr Devices Emax, Sunnyvale, CA, USA). Enzyme activity was expressed as the fold increase in the proportion of apoptotic cells over that of non-treated control cells.
Total RNA was isolated from the HIT-T15 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instruction. Equal amounts of total RNA was reverse transcribed to cDNA using ImProm-II™ reverse transcriptase (Promega, Madison, WI, USA) and oligo(dT)15 primer (Promega). Real-time PCR was conducted using DNA Engine Opiticon System (MJ Research Inc., Watertownm, MA, USA) in 20 µL reaction mixture containing 10 µL of SYBR Premix Ex Taq (Takara Bio Inc., Otsu, Japan), 2 pM of forward primer, 2 pM of reverse primer, and 1 µg of cDNA. Amplification parameters consisted of an initial denaturation at 95℃ for 5 min and 40 cycles of three-step PCR (a denaturation at 95℃ for 1 min, an annealing at 55℃ for 30 sec, and an extension at 72℃ for 1 min). Expression levels of the house keeping gene GAPDH were used to control for differences in loading and cDNA synthesis efficiency between samples. The expression levels of Insulin2 mRNA were displayed as the cycle of threshold (Ct) value normalized to the GAPDH mRNA value. To determine the relationship between Ct values and mRNA levels, primers were calibrated by using serial dilution of cDNA. We confirmed good correlations between Ct values and cDNA dilutions in both primer sets. We used primer for Insulin2 (5'-TGTGGTTCTCACTTGGTGGA-3' 5'-GCTCCAGTTGTGCCACTTGT-3') and GAPDH (5'-AAGTTCAACGGCACAGTCAA-3' 5'-TACTCAGCACCAGCATCACC-3').
Whole cell lysates were prepared by lysis cells in Proprep-protein extraction solution (Intron Biotechnology, Seoul, Korea) containing 10 mM sodium phosphate (pH 7), 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 150 mM NaCl, 50 mM NaF, 0.1 mM sodium vanadate, 4 µg/mL leupeptin, 1 mM PMSF, and protein concentration of the lysates were measured with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins (20 µg) were separated on a 4-20% SDS-PAGE and transferred by electroblotting to a nitrocellulose membrane. The membranes were washed with PBST containing 5% nonfat dry milk at room temperature and incubated for 2 hr with the primary antibodies under the same conditions. The following primary antibodies were used: anti-JNK, anti-Thr183/Tyr185 phosphospecific JNK, anti-caspase-9, anti-caspase-3 (Cell signaling, Beverly, MA, USA); anti-Akt antibody, anti-Ser473 phosphospecific Akt, anti-GSK3β, anti-Ser9 phosphospecific GSK3β (Upstate, Lake Placid, NY, USA); anti-β-actin (for internal control) (Sigma, St. Louis, MO, USA). The membranes were washed in PBST, and incubated for 1 hr with horseradish peroxidase-conjugated sheep anti-mouse and donkey anti-rabbit immunoglobulin antibody (1:500) (Amersham Pharmacia Biotechnology, Tokyo, Japan) under the same conditions. After washing with PBST, the specific signals were detected with an enhanced chemiluminescense detection system (Amersham Pharmacia Biotechnology, Tokyo, Japan). Reagents for electrophoresis were obtained from Bio-Rad Laboratories (Hercules, CA, USA).
Insulin concentrations in the human islet culture medium were measured with the human ELISA insulin kit (Linco Research, St. Charles, MO, USA) according to the manufacturer's instructions and insulin concentrations were calculated using the microplate reader (Molecualr Devices Emax, Sunnyvale, CA, USA).
Experiments were separately conducted at least three times, and data are expressed as the means±SE. The statistical differences were analyzed using the one-way ANOVA followed by Turkey test. The SPSS statistical software package (Version 14.0, Chicago, IL, USA) was used for the statistical analysis.
To determine the time and dose conditions in β-cell apoptosis by oxidative stress, we performed the MTT assay. HIT-T15 cells treated with H2O2 (100 µM) had been shown to reduce cell viability in a time-dependent manner (Fig. 1A). The cell viability decreased to ~50% when cells were treated with 100 µM H2O2 for up to 2 hr. Then, we investigated the effects of the Ex-4 on oxidative stress-induced apoptosis. As seen in Fig. 1B, MTT assay shows that treatment of the cells with Ex-4 significantly increased oxidative stress-reduced cell viability. Also, we evaluated apoptotic nuclear condensation and fragmentation of HIT-T15 cells after Ex-4 and NAC treatment under oxidative stress (Fig. 1C). The morphological observations of H2O2 treated cells revealed marked chromatin condensation and the formation of apoptotic bodies, whereas the treatment of Ex-4 or NAC in these conditions reduced apoptotic bodies. In order to further clarify these results, we investigated protective effect of Ex-4 through flow cytometric analysis (Fig. 1D). As expected, the Ex-4 or NAC treatment reduced H2O2-induced apoptosis. For example, the apoptosis ratio of the control and those treated with H2O2 were 9.4% and 45.5%, respectively, when treated Ex-4 or NAC, the apoptosis ratio was 3.8% or 10.2%, respectively. These data suggested that pretreatment of Ex-4 protect from oxidative stress-induced apoptosis.
To investigate the mechanism of the cell protective effect induced by GLP-1 under oxidative stress, we examined that the protective effect of Ex-4 in oxidative stress-activated apoptosis was associated with PI3-kinase/Akt/GSK3β and phospho-JNK reduction in human islet (Fig. 2) and HIT-T15 cells (data not shown). Fig. 2A shows that H2O2 treatment reduced phospho-Akt (Ser 473) and phospho-GSK3β (Ser-9), but pretreatment of NAC, Ex-4 or forskolin maintained or increased its phosphorylation. Also, exposure of human islet to H2O2 activated JNK through its phosphorylation. In these conditions, pretreatment of Ex-4 or forskolin blocked JNK phosphorylation. The PKA inhibitor H89 abolished Ex-4 and forskolin effects through decreased phospho-Akt (Ser 473) and phospho-GSK3β (Ser-9) and increased phospho-JNK. Next, we found that JNK inhibition by SP600125, JNK inhibitor, resulting from increased GSK3β (Ser-9) phosphorylation (Fig. 2B). These data demonstrated that the oxidative stress-induced apoptosis might be associated with PI3-kinase/Akt/GSK3β and JNK signaling pathway, and suggested that Ex-4 protects GSK3β induced and JNK mediated apoptotic pathway.
For apoptosis to proceed, the activation of a family of caspases plays a pivotal role. Caspases-8 and -9, as initiators, are activated through self-processing, and cleave downstream procaspase-3 to the active form of caspase-3, which functions as the executor of apoptosis (23). To determine whether apoptosis via JNK and PI3-kinase/Akt/GSK3β ultimately is induced the activation of these caspases, HIT-T15 cells were exposed to oxidative stress and the levels of caspases-8, -9 and caspase-3 were examined with regard to enzyme activity using a colorimetric assay and western blot (Fig. 3). As shown in Fig. 3A, H2O2-induced apoptosis increases the activity of caspase-9 and -3 by 2.1- and 3.4-fold, respectively compared with the controls, whereas pretreatment of Ex-4 or NAC in these conditions reduced the activity of caspase-9 and -3. H89 abolished the attenuation of caspase-9 and -3. However, H2O2 did not induce the activation of caspase-8. Likewise, the active form of caspase-9 and -3 were expressed in H2O2-treated cells and reduced in Ex-4 or NAC treated cells (Fig. 3B). Thus, β-cell apoptosis through JNK and PI3-kinase/Akt/GSK3β in oxidative stress condition finally is induced caspase activity, and this apoptotic activity is involved a mitochondrial pathway. Also, β-cell-protective effects of Ex-4 or NAC is induced through inhibition of the caspase activity by mitochondrial pathway in these conditions.
Finally, to investigate whether Ex-4 increases the practical ability of insulin secretion, we performed change of insulin2 mRNA in β-cell line and insulin secretion in human islet. As shown in Fig. 4, under H2O2-treatment condition, expression of insulin2 mRNA and insulin secretion were decreased remarkably, but treatment of NAC or Ex-4 in oxidative stress was recovered dramatically.
The long-acting GLP-1R agonist Ex-4 represents a new promising class of drugs for treatment of diabetes. In our current study, we have demonstrated that the protective effect and mechanism of Ex-4 in oxidative stress-induced β-cell apoptosis. GLP-1 and GLP-1R agonist Ex-4 enhance insulin release, increase insulin biosynthesis, promote β-cell development/replication, and prevent β-cell exhaustion under the diabetic conditions (17-19). Also, GLP-1 and GLP-1R agonist Ex-4 may have an antiapoptotic effect on β-cell (20), but detailed mechanisms are not proven.
It has been suggested that various pathways which cause β-cell damage participate in the hyperglycemia induced apoptosis in β-cell damage (5, 10). There is considerable evidence that hyperglycemia results in the generation of reactive oxygen species (ROS), ultimately leading to increased oxidative stress, and chronic hyperglycemia triggers the development the late complications of diabetes (1, 2, 5). Also, β-cells are considered to be very sensitive to oxidative stress (9, 10), and resultant apoptotic cell death was reported to occur in diabetic model animals (9, 24).
Under diabetic conditions, oxidative stress is induced in various tissues, leading to activation of the JNK pathway (9, 10). Activation of this pathway is involved in the deterioration of pancreatic β-cell function (9, 10). Inhibition of either the PKA or PI3-kinase/Akt/GSK3β pathway, the two major pathways through which Ex-4 triggers its anti-apoptotic effects, showed that PKA and PI3-kinase/Akt/GSK3β was responsible for Ex-4 mediated blocking of the JNK signaling. Incubation of the human islet β-cells with forskolin, activators of the cAMP/PKA pathway, mimicked the effects of Ex-4 on the JNK phosphorylation (Fig. 3). Also, H89, a pharmacological inhibitor of the PKA, efficiently antagonized the effects of forskolin on the JNK activity (Fig. 3). In this report, we showed that the protection mediated by Ex-4 is associated with an inhibition of the JNK pathway. Indeed, we found that JNK inhibition in human islet β-cells treated with SP600125 specifically stimulated the phosphorylation of GSK3β (Fig. 3). GSK3β is a well-characterized downstream target of growth factor-activated PI3-kinase/Akt signaling (14, 15). We showed that oxidative stress is attenuated Akt phosphorylation and resultant dephosphorylation of GSK3β. Ex-4 treatment reduced ER stress-induced apoptosis and was also associated with reductions in the dephosphorylation of Akt and GSK3β. The conclusion is that GSK3β by JNK activation modulates susceptibility to oxidative stress-induced apoptosis, rather than its activity being altered by apoptosis or impaired insulin secretion, was shown by reduction of GSK3β expression. Several transcription factors are potential targets whereby this kinase could promote apoptosis (25). For example, CREB upregulates the expression of the antiapoptotic protein Bcl-2, and the inhibition of CREB activity by GSK3β may contribute to the proapoptotic effects of GSK3β (26, 27). Moreover, β-cell apoptosis via JNK and PI3-kinase/Akt/GSK3β in oxidative stress condition ultimately is induced by caspase activity involved a mitochondrial pathway (Fig. 4). However, the precise proapoptotic targets of GSK3β through JNK activation during oxidative stress in β-cell remain to be identified.
JNK and Akt/GSK3β inhibition by Ex-4 on oxidative stress-induced apoptosis leads to improved insulin sensitivity. Our study investigated that Ex-4 increased the practical ability of insulin secretion, through increase of insulin2 mRNA in β-cell line and insulin secretion in human islet by Ex-4 treatment on oxidative stress condition. Thus it is possible that protective action of Ex-4 against oxidative stress could be involved as a treatment for type 2 diabetes.
In conclusion, we showed that the potent inhibitory effect of GLP-1/cAMP/PKA system on the JNK and PI3-kianas/Akt/GSK3β activity as a major mechanism for preventing β-cell apoptosis induced by oxidative stress. Further studies will likely clarify the signaling pathways from oxidative stress to PI3-kinas/Akt/GSK3β and will identify GSK3β downstream targets responsible for apoptosis. This mechanism largely contributes to the efficiency of Ex-4 in the long time preservation of β-cells against apoptosis in the treatment of type 2 diabetes.
Figures and Tables
Fig. 1
Effects of the NAC or Ex-4 on oxidative stress-induced apoptosis. HIT-T15 cells were pretreated NAC (5 mM) or Ex-4 (25 nM), for 1 hr before stress induction. (A) After exposure to 100 µM H2O2, HIT-T15 cells apoptosis increased by time. Cells viability was measured with the MTT reduction assay. (B) After treatment of H2O2 (100 µM, 2 hr), effects of the NAC or Ex-4 on cell viability were measured by MTT reduction assay. (C) H2O2 (100 µM, 4 hr)-induced apoptotic nuclei reduced via NAC or Ex-4. Photographs were taken using a blue filter at a magnification of ×400. (D) Flow cytometric analysis of apoptosis of HIT-T15 cells exposed to H2O2 (100 µM, 8 hr). Apoptotic cells were measured by FACS analysis after Annexin V/PI staining. FL1: Annexin V-FITC, FL2: PI. Data are shown as the means±SE of six independent experiments.
*P<0.001 vs control cells; †P<0.001 vs H2O2 alone.

Fig. 2
Protective effect of Ex-4 on oxidative stress-induced apoptosis via PI3-kinase/Akt/GSK3β and JNK signaling pathway in human islet. Human islet was pretreated with H89 (PKA inhibitor, 10 µM) or SP600125 (JNK inhibitor, 20 µM). After 30 min, human islet was treated Ex-4 (25 nM), NAC (5 mM) or forskolin (20 µM), for 1 hr before stress induction. After treatment of H2O2 (100 µM, 2 hr), (A) In oxidative stress condition, expression change of total Akt, GSK3β, JNK, phospho-Akt (Ser 473), phospho-GSK3β (Ser-9) and phospho-JNK were assessed by western blot. (B) Total JNK, GSK3β, phospho-GSK3β (Ser-9) and phospho-JNK were detected in the absence or presence of SP600125. These results are representative of three independent experiments.

Fig. 3
Protective effect of Ex-4 on caspase-8, 9, and -3 activity in oxidative stress-induced apoptosis. HIT-T15 cells were pretreated with H89 (PKA inhibitor, 10 µM). After 30 min, HIT-T15 cells were treated Ex-4 (25 nM) or NAC (5 mM), for 1 hr before stress induction. After treatment of H2O2 (100 µM, 2 hr), (A) Cell lysates were incubated with IETD-pNA, LEHD-pNA and DEVD-pNA for in vitro caspase-8, -9, and -3, respectively. The caspase-8, -9, and -3 activity were measured by the colorimetric assay. (B) Active form of capase-9 and -3 were detected using western blot analysis. Data are shown as the means±S.E. of three independent experiments.
*P<0.01 vs control cells; †P<0.01 vs H2O2 alone; ‡P<0.01 vs Ex-4 or NAC in treated H2O2.
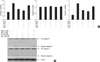
Fig. 4
Effects of the Ex-4 on insulin secretion. Human islet was pretreated with H89 (10 µM). After 30 min, HIT-T15 cells or human islet was treated Ex-4 (25 nM) and NAC (5 mM) for 1 hr before stress induction. After treatment of H2O2 (100 µM, 2 hr), (A) Expression levels of insulin2 mRNA were examined by real time RT-PCR in HIT-T15 cells. Data were expressed as the rates to the expression levels to GAPDH in the same sample. GAPDH used for loading control. (B) Insulin secretion in human islet was detected by Insulin ELISA kit. Data are shown as the means±S.E. of four independent experiments.
*P<0.01 vs H2O2 alone; †P<0.01 vs Ex-4 in treated H2O2.
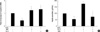
Notes
This work was supported by the National Research Foundation of Korea Grant funded by Korea Government (KRF-2007-313-E00228) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R11-2008-044-03003-0). The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999. 274:14112–14121.
2. Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008. 9:329–343.
3. Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000. 23:1084–1091.
4. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.

5. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002. 23:599–622.

6. Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death. Science. 2005. 307:380–384.
7. King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am. 1996. 25:255–270.

8. Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001. 17:189–212.
9. Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Matsuhisa M, Yamasaki Y. Oxidative stress and the JNK pathway in diabetes. Curr Diabetes Rev. 2005. 1:65–72.

10. Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M, Yamasaki Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med. 2005. 83:429–439.

11. Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002. 277:30010–30018.

12. Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001. 7:1133–1137.
13. Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001. 108:1631–1638.
14. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995. 378:785–789.

15. Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007. 282:12030–12037.

16. Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008. 51:623–631.
17. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007. 113:546–593.

18. Park IB. New and emerging drugs in type 2 diabetes. Korean J Med. 2007. 72:446–450.
19. Kim BJ. Stimulation of glucagon like peptide-1 secretion in enteroendocrine L cells. Korean Diabetes J. 2009. 33:458–463.

20. Kim JY, Lee SK, Baik HW, Lee KH, Kim HJ, Park KS, Kim BJ. Protective effects of glucagon like peptide-1 on HIT-T15 beta cell apoptosis via ER stress induced by 2-deoxy-D-glucose. Korean Diabetes J. 2008. 32:477–487.
21. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000. 343:230–238.

22. Steensma DP, Timm M, Witzig TE. Flow cytometric methods for detection and quantification of apoptosis. Methods Mol Med. 2003. 85:323–332.
23. Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002. 7:313–319.
24. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008. 118:3378–3389.
25. Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001. 359:1–16.

26. Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000. 275:10761–10766.

27. Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem. 2001. 78:1219–1232.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download