Abstract
The massive reorganization of microtubule network involves in transcriptional regulation of several genes by controlling transcriptional factor, nuclear factor-kappa B (NF-κB) activity. The exact molecular mechanism by which microtubule rearrangement leads to NF-κB activation largely remains to be identified. However microtubule disrupting agents may possibly act in synergy or antagonism against apoptotic cell death in response to conventional chemotherapy targeting DNA damage such as adriamycin or comptothecin in cancer cells. Interestingly pretreatment of microtubule disrupting agents (colchicine, vinblastine and nocodazole) was observed to lead to paradoxical suppression of DNA damage-induced NF-κB binding activity, even though these could enhance NF-κB signaling in the absence of other stimuli. Moreover this suppressed NF-κB binding activity subsequently resulted in synergic apoptotic response, as evident by the combination with Adr and low doses of microtubule disrupting agents was able to potentiate the cytotoxic action through caspase-dependent pathway. Taken together, these results suggested that inhibition of microtubule network chemosensitizes the cancer cells to die by apoptosis through suppressing NF-κB DNA binding activity. Therefore, our study provided a possible anti-cancer mechanism of microtubule disrupting agent to overcome resistance against to chemotherapy such as DNA damaging agent.
The nuclear factor κB (NF-κB) is a rapidly inducible transcription factor involved in regulating the expression of genes relevant in wide range of different cellular processes including apoptosis, cell survival and immune response (1, 2). In most types of cells, inactive NF-κB is retained in the cytoplasm through its interaction with the inhibitory proteins known as IκBs (1). Upon activation by various stimuli, such as proinflammatory cytokines, ionizing radiation (IR), DNA damaging agents and cellular stress, IκBs are phosphorylated by the IκB kinase (IKK) complex, and rapidly degraded by the proteasome after polyubiquitination, resulting in NF-κB translocation into the nucleus (3, 4). Accumulating evidence indicates that NF-κB plays a critical role in cellular protection against to various anticancer drugs- or IR-induced apoptosis, and therefore suggested that the activation NF-κB may contribute in blocking the efficacy of cancer therapies and radiation (5-7). Indeed, inhibition of NF-κB leads to sensitization of apoptotic cell death in response to IR or DNA damaging agents in large number of cancer cells (8-10). Furthermore, aberrant dysregulation of NF-κB activity or increased nuclear level of NF-κB subunit (RelA/p50) has been commonly observed in several solid tumors and hematopoietic malignancies, and chemoresistance in selected tumors has been linked to the activated NF-κB status (11-13). Therefore, selective suppression of NF-κB will provide a useful strategy for overcoming drug-resistance in patients treated with conventional chemotherapy.
In addition to DNA-damaging agents, it has been previously reported that massive microtubule reorganization by microtubule disrupting agents such as cytochalasins or nocodazole activates NF-κB and induces NF-κB dependent gene expression (14, 15). So far, although the exact molecular mechanism by which microtubule disrupting agents leads to activation of NF-κB remains to be elucidated, the sensing changes in the state of cytoskeleton may be one of way to regulate NF-κB signaling pathway. Relevant to this issue, microtubule system has recently been identified as an important regulator of translocation of the active NF-κB from cytosol to the nucleus (16, 17). Thus, it is possible that the disruption of microtubule could be important regulator for DNA damage-induced NF-κB activation, or acts in synergy to sensitize the apoptotic response against to chemotherapeutic agents.
Here we report that disruption of microtubule inhibits DNA damage-induced NF-κB binding activity without alteration of nuclear translocation of NF-κB subunit, even though it can enhance NF-κB activation in the absence of other signals. Furthermore, such suppressed NF-κB binding activity by combined treatment of microtubule disrupting agent was correlated with the sensitization effect of apoptotic response to DNA damage agents. These results support the idea that, the combined treatment of these compounds could have a synergic potential use in cancer therapy to overcome NF-κB mediated resistance.
Human cervical cancer epithelial cells HeLa (American Type Culture Collection, CCL-2, Bethesda, MD, USA) and mouse embryonic fibroblasts (MEF) cells, including wild-type and p65-/-cells (kindly donated by Dr Zhengang Liu; Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, antibiotics (100 U/mL penicillin G and 100 µg/mL streptomycin), and 10% heat-inactivated FBS and were maintained at 37℃ in a humidified incubator containing 5% CO2.
All commercial antibodies were purchased from the following: anti-IKKβ (Upstate Biotech, Waltham, MA, USA); anti-p84N5 (GeneTex, Irvine, CA, USA); anti-actin (Sigma-Aldrich, St. Louis, MO, USA); anti-phospho-IκB-α, anti-caspase-3 and anti-caspase-9 (Cell Signaling Technology, Beverly, MA, USA); anti-IκB-α, anti-p65, anti-tubulin, anti-SP1 (Santa Cruz Biothenology, Santa Cruz, CA, USA), anti-poly (ADP-ribose) polymerase (PARP) (BD Biosciences Pharmingen, San Diego, CA, USA). Cochicine (Col) were purchased from Sigma-Aldrich. Trypan blue was purchased from Cambrex Bio Science (Walkersville, MD, USA). Adriamycin (Adr), camptothcin (Cpt), nocodazole (Noc), vinblastine (Vin), the NF-κB inhibitor BAY-11, the selective inhibitor of IKK-β2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3- thiophenecarboxamide (TPCA-1) and the pancaspase inhibitor Z-VAD-FMK were purchased from Calbiochem (San Diego, CA, USA). Poly(dI-dC)·poly(dI-dC) and dNTP were purchased from Pharmacia LKB Biotechnology. [α-32P]dCTP was purchased from Amersham (Piscataway, NJ, USA).
After treatment with different reagents as described in the Figure legends, cells were collected and lysed in M2 buffer (20 mM Tris at pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM DTT, 0.5 mM PMSF, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, 1 µg/mL leupeptin). Fifty micrograms of the cell lysates were subjected to SDS-polyacrylamide gel and blotted onto PVDF membrane. After blocking with 5% skim milk in PBS/T, the membrane was probed with the relevant antibody and visualized by enhanced chemiluminescence (ECL), according to the manufacturer's instruction (Amersham).
Nuclear extracts from whole cell extracts were prepared as described elsewhere (18), with minor modifications. In brief, after treatment with different reagents as described in the figure legends, cells were centrifuged (1,500 rpm, 5 min, 4℃) and suspended in ice-cold buffer A (10 mM HEPES pH 7.9; 10 mM KCl; 0.1 mM EDTA; 0.1 mM EGTA; 1 mM DTT; 0.5 mM phenylmethanesulfonyl fluoride [PMSF]) and 0.5% NP-40 for 30 min, and then centrifuged (12,000 rpm, 10 min, 4℃) to separate the cytosolic fraction (supernatant) and nucleus fraction (pellet). The nucleus fraction was re-suspended in ice-cold buffer C (20 mM HEPES, pH 7.9; 400 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM DTT; 1 mM PMSF; 20% glycerol) for 30 min, and centrifuged at 12,000 rpm for 10 min, 4℃. Protein concentration was estimated using the Bio-Rad protein assay reagent and an equal amount of proteins per sample of nuclear extract was further analyzed by SDS-PAGE and EMSA.
EMSA was performed as described previously (18). Briefly, 5 µg of nuclear extract was incubated at room temperature for 20 min with reaction buffer containing 20 mM HEPES, pH 7.9, 50 mM KCl, 0.1 mM EDTA, 1 mM DTT, 5% glycerol, 200 µg/mL BSA, and 2 µg of poly(dI-dC)·poly(dI-dC). Then the 32P-labeled double-stranded oligonucleotide (1 ng, ≥1×105 cpm) containing the NF-κB binding consensus sequence (5'-GGCAACCTGGGGACTCTCCCTTT-3') was added to the reaction mixture for an additional 10 min at room temperature. The reaction products were fractionated on a nondenaturating 6% polyacrylamide gel, which was then dried and subjected to autoradiography. Western blot analysis with antibody against SP1 was also performed in the same nuclear extract as a control protein input.
Cells were seeded in six-well plates and treated with the indicated concentrations of reagents as described in the figure legends. Cell death, as observed by microscopy, was characterized by rounding up and detaching of the cells from the plates. Cell death was quantified by trypsinization, followed by staining with trypan blue and counting with a hemocytometer (American Optical, New York, NY, USA). The stained cells (blue) were counted as dead cells and were expressed as a percentage of cells. For each treatment, triplicate experiments were performed.
Data are expressed as the mean±S.E. from at least three separate experiments performed. The differences between groups were analyzed using a Student's t test, and P<0.05 is considered statistically significant. Statistical analyses were carried out using SPSS software (ver. 11.0; SPSS Inc., Chicago, IL, USA).
To characterize the NF-κB signaling pathway in HeLa cells affected by the disruption of the microtubule network, we compared the kinetics of the degradation of IκB-α at various time points after tumor necrosis factor (TNF) or tubulin polymerization inhibitors (colchicine, vinblastine and nocodazole). As shown in the bottom panel of Fig. 1A, when cells were treated with TNF, the expression of IκB-α started to be degraded at 15 min after treatment, and was recovered by 60 min. In contrast to the cells treated with TNF, the IκB-α degradation and its recovery were slower and weaker upon treatment with the microtubule disrupting agents (top three panels of Fig. 1A), which suggest that disruption of the microtubule network affects NF-κB activity in a different manner with a classical pathway. Since the nuclear translocation of NF-κB subunit, relA (p65) is essential for the NF-κB signaling pathway, we next examined the effect of the microtubule disrupting agents on the nuclear translocation of p65. As expected, the treatment of microtubule disrupting agents resulted in enhanced translocation of p65 into the nucleus, even though the extent of accumulated p65 into nucleus was lower than that of TNF treatment (Fig. 1B). As previously reported (19), the expression of nuclear protein encoded by N5 gene (p84N5) occurred principally in the nuclear fraction (Fig. 1B, bottom panels). However, the expression levels of p65 on the total cell extract were unaffected by treatment with microtubule disrupting agents (Fig. 1A). To confirm these observations, nuclear extracts were examined for NF-κB binding activity by EMSA using a palindromic NF-κB binding site. Consistent with above results, the binding activity of NF-κB subunit complex was gradually increased in response to microtubule agents, indicating that microtubule disruption directly induce NF-κB binding activity through nuclear translocation of NF-κB subunit, p65 without the expressional change of NF-κB. As a control of nuclear protein contents, the SP-1 protein level was examined by immunoblotting with an anti-SP-1 antibody and no difference was observed (Fig. 2B, bottom panels).
Previous studies have demonstrated that the DNA damage trigger a cytoplasmic signaling that leads to the activation of IKK and NF-κB, which is associated predominantly with protecting cells from apoptosis (6, 7). Therefore it is a possibility that NF-κB activation by DNA damaging agents could be responsible for the development for the chemoresistance. We next examined whether the DNA damage-induced NF-κB activation is affected by the disruption of microtubule network. Two DNA damaging agents, adriamycin (Adr) and comptothecin (Cpt), were used to detect the degradation of IκB-α and NF-κB binding activity in total cell extract and nuclear extract respectively. As similar to the effect of microtubule disrupting agents, Adr and Cpt induced a slow and sustained degradation of IκB-α protein until after 9 h of treatment (Fig. 2A). Consistently, an increase in NF-κB binding activity in response to Adr or Cpt treatment was evident, and the kinetics of NF-κB binding activity were correlated well with the IκB-α degradation (Fig. 2B, top panels).
Recent reports (16, 17) support the notion that microtubule network may play an important role for nuclear translocation of NF-κB subunits even though the microtubule disruption paradoxically induced NF-κB activation (Fig. 1). To examine the issue whether microtubule integrity affect the DNA damage-induced NF-κB activation, we evaluated the NF-κB binding activity at different time points under the condition of microtubule disruption. Interestingly, pretreatment with Col, Vin or Noc dramatically suppressed either Adr- or Cpt-induced NF-κB binding activities (Fig. 3), indicating that functional microtubule network is required for the DNA binding activity. We also confirmed that TNF-induced NF-κB DNA binding activity was suppressed by pretreatment with these microtubule disrupting agents (Data not shown).
Because the above results clearly demonstrated that microtubule disrupting agents suppressed the DNA damage-induced NF-κB DNA binding activity, we subsequently examined whether these agents affect the IκB-α degradation and nuclear translocation of p65 induced by DNA damage. Surprisingly, there was detectable degradation of IκB-α protein in response to Adr- or Cpt treatment under the condition of microtubule disruption (Fig. 4A, B). Furthermore pretreatment with Col, Vin or Noc failed to block the Adr- or Cpt-induced nuclear translocation of p65 (Fig. 4C, D). These data suggest that microtubule assembly has an important role for DNA damage-induced NF-κB binding activity at the level of nucleus but not at the cytoplasmic event.
Previous study has reported that DNA damaging agents such as Adr or Cpt can directly induce apoptosis in human cancer cells (20). The nature of DNA damage-induced cell death was assessed by the activation of the caspase cascade, including caspase-9 and -3 and resultant cleavage of PARP. Caspase-9, -3 and PARP were processed after Adr treatment, as shown by the degradation of inactive pro-enzymes in a time-dependent manner (Fig. 5A, top three panels). Moreover, the increased processing of caspase cascade by Adr was completely abolished by pretreatment with Z-VAD-FMK, a membrane permeable pancaspase inhibitor (Fig. 5A, fifth row), indicating that the activation of caspase cascade plays an important role in DNA damage-induced apoptotic cell death in HeLa cell. Because NF-κB has been implicated in apoptosis, we further examined whether the activation of NF-κB plays a role in DNA damage-induced cell death by comparing the responses of wild-type (WT) and p65-null (p65-/-) MEF cells. As shown in Fig. 5B, the extent of cell death was much higher in p65-/- MEF cells than in WT MEF cells measured by trypan blue exclusion assays. To ensure that p65 expression is indeed defective in p65-/- MEF cells, the expression levels of p65 and IKK-β in WT and p65-/- MEF cells are examined (Fig. 5C). Furthermore, pretreatment of HeLa cells with NF-κB-specific inhibitor Bay-11 and TPCA-1 before Adr exposure dramatically enhanced Adr-induced cell death (Fig. 5D). These results clearly indicate that NF-κB activation provides an important anti-apoptotic role in DNA damage-induced cell death.
To determine whether inhibition of NF-κB binding activities by microtubule disruption leads to sensitization of cytotoxic response against DNA damaging agent, HeLa cells were pretreated with microtubule disrupting agents for 30 min followed by Adr, and cell viability was observed with a inverted light microscope and trypan blue exclusion assay. As shown in Fig. 5E, pretreatment with Col, Vin and Noc markedly enhanced Adr-induced cytotoxicity, whereas only a limited cells death was observed in Z-VAD-FMK pretreated cells. The quantification of time-dependent cell death was summarized in Fig. 5F. Data suggested that microtubule disruption greatly sensitized DNA damage-induced apoptotic cell death. Furthermore, the combined treatment of microtubule disrupting agents and Adr significantly enhanced the cleavage of both caspase-9 and casapse-3 as evidenced by the decrease of procaspase-9 and increase of the active forms of caspase-3 (p21 fragment) (Fig. 5G, top two panels). A time-dependent enhancement of PARP cleavage was also detected in cells co-treated with microtubule disrupting agents and Adr (Fig. 5G, third panel). Thus these results indicate that microtubule disrupting agents are able to sensitize DNA damage-induced apoptosis via promoting the caspase cascade.
It has been well established that the activity of NF-κB can be induced by IR or some conventional chemotherapeutic agents including Adr, etoposide, Cpt, vinca alkaloids and taxol (4, 15). Once activated by those stress, NF-κB has been shown to suppress the apoptotic potential of the chemotherapy in several cancer cells and in vivo cancer model (21, 22). Thus it has been widely proposed that activation of NF-κB in response to chemotherapy could be a potential mechanism of inducible chemoresistance which protects cancer cells from apoptotic stress. As documented previously, anti-carcinogenic property of DNA damaging agents such as Adr and Cpt is closely associated with an activation of tumor suppressor gene p53 which results in limited cell proliferation and mitochondrial dependent apoptosis through transcriptionally activating p53 target genes (23, 24). In accordance with previous observations in a variety of tumor cell lines, we found that Adr-induced apoptosis in HeLa cells appeared to accompany the activation of caspase-9 and -3, and subsequent PARP cleavage (Fig. 5A), which indicate a prominent role for mitochondria in Adr-induced cell death. In the intrinsic mitochondrial apoptotic pathway, p53-target gene products including Bax and Bak are involved in the release of cytochrome c, resulting in activation of caspase-9, which in turn activates execution-type caspase-3 (25, 26). In contrast to p53-mediated apoptotic response, DNA damaging agents also activates NF-κB signaling pathway which can suppress mitochondrial-dependent apoptotic pathway through transcriptional induction of anti-apoptotic genes including Bcl2. In addition, a number of factors including changes in cellular levels of P-glycoprotein family of multi- drug resistance (MDR), detoxification enzymes (e.g., glutathione S-transferase), DNA repair enzymes (e.g., DNA topoisomerase II), various oncogenes and mutated p53 have associated with development of cancer chemoresistance (27, 28). In this study, NF-κB subunit (p65) null MEF cells were found to be much more resistant to cell death as compared with wild-type cells, which suggest that NF-κB plays a role in blocking the efficacy of chemotherapy with DNA damaging agents (Fig. 5B). This interpretation is further supported by our observation that the inhibition of NF-κB using Bay-11 and TPCA-1 led to a dramatic enhanced cell death induced by Adr in HeLa cells (Fig. 5D). Although the further studies will be required to elucidate the exact molecular mechanisms of cancer chemoresistance, our study clearly raises the possibility that NF-κB could functions as an anti-apoptotic mediator in response to DNA damage. Consistent with this conclusion, there are some evidences that the combinational chemotherapies to inhibit NF-κB activation, including the administration of proteasome-specific inhibitors such as velcade, MG132 or some antioxidants such as N-acetylcystein provide a chemosensitization against DNA damage-induced killing (5).
In addition to DNA damaging agents, previous studies reported that microtubule disrupting agents can enhance the NF-κB dependent transcriptional activity presumably through the increased activity of protein kinase C (14, 29). Consistently we have also confirmed that Col, Vin and Noc have all been shown to activate NF-κB (Fig. 1). However, it has been recently proposed that the state of microtubule polymerization may also be crucial in the translocation of the active NF-κB subunit from cytosol to the nucleus. For instance, disruption of microtubule function by pretreatment with Col or Vin impaired accumulation and subsequent binding activities of NF-κB subunit p65 and p50 in the nucleus following glutamate stimulation (30) or zinc deficient cells which caused deficit to tubulin polymerization (16). Based on these observations, we speculated that the combined treatment of microtubule disrupting agents affected the nuclear targeting of NF-κB by DNA damage. Interestingly, nuclear translocation of NF-κB subunit p65 in response to DNA damage was not affected after pretreatment with microtubule disrupting agents in our experimental conditions (Fig. 4C, D). Similarly, Mikenberg et al. (30) have shown that microtubule perturbing compounds did not affect TNF-induced nuclear accumulation of p65 by using confocal microscopy. These results strongly suggest that NF-κB movement activated by either DNA damage or TNF is independent of cytoskeleton-based transport system in non-neuronal cancer cells. Despite these insights, we found that the microtubule disruption with Col, Vin and Noc led to drastically suppressed DNA damage-induced NF-κB binding activity by EMSA using nuclear extracts (Fig. 3), suggesting that the functional microtubule network is required for the DNA binding activity rather than the nuclear translocation of activated NF-κB subunit p65. Furthermore, we also found a significant increase in caspase-dependent apoptotic cell death after Adr treatment under the condition of microtubule disruption (Fig. 5E-G), suggesting that the suppressed NF-κB binding activity by the combined therapy may contribute to sensitize to trigger apoptotic machinery.
Taken together, data from this study collectively suggest that inhibition of microtubule network significantly chemosensitizes the cancer cells to die by apoptosis through suppressing NF-κB DNA binding activity. However, further study elucidating the molecular mechanism of microtubule disrupting agents on the regulation of NF-κB DNA binding activity in the nucleus will be critical for improving the value in the clinical application of these combined chemotherapies.
Figures and Tables
Fig. 1
Microtubule disrupting agents induces NF-κB activation through nuclear translocation of NF-κB subunit p65. (A) HeLa cells were treated with microtubule disrupting agents (upper panel) including colchicines (Col, 10 µM), vinblastine (Vin, 1 µM) and nocodazole (Noc, 0.5 µM) or TNF (lower panel) for various times as indicated. Cell extracts were applied to SDS-PAGE for immunoblotting with anti-IκB-α and anti-p65 antibodies. As a protein loading control, the same amounts of extracts were analyzes by immunoblotting with anti-IKK-β antibody. (B, C) After HeLa cells were treated with 10 µM of Col, 1 µM of Vin or 0.5 µM of Noc for various times as indicated, nuclear extracts were obtained as described under Materials and Methods. (B) The nuclear translocation of NF-κB subunit p65 was analyzed by immunoblotting with anti-p65 and anti-p84N5 antibodies from nuclear extracts from each sample. (C) EMSAs were performed with labeled NF-κB oligomers and 5 µg of nuclear extracts to analyze NF-κB binding activity. As a control, 50 µg of the nuclear extracts were applied to SDS-PAGE for immunoblotting with anti-SP1 antibody.

Fig. 2
DNA damaging agents induces NF-κB activation. (A) HeLa cells were treated with adriamycin (Adr, 10 µg/mL) or comptothecin (Cpt, 100 µM) for various times as indicated. Cell extracts were applied to SDS-PAGE for immunoblotting with anti-IκB-α and anti-IKK-β antibodies. (B) HeLa cells were treated as described in A for various times as indicated. Nuclear extracts were prepared and 5 µg of nuclear extracts from each sample was used to analyze NF-κB binding activity by EMSA with a NF-κB probe.

Fig. 3
Microtubule disrupting agents suppresses the DNA damage-induced NF-κB binding activity. HeLa cells were treated with Adr (10 µg/mL) or Cpt (100 µM) for various times as indicated in the presence or absence of Col (A, 10 µM), Vin (B, 1 µM) and Noc (C, 0.5 µM). Nuclear extracts were prepared and 5 µg of nuclear extracts from each sample was used to analyze NF-κB binding activity by EMSA as described in Fig. 2B. As a control, 50 µg of the nuclear extracts were applied to SDS-PAGE for immunoblotting with anti-SP1 antibody.
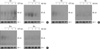
Fig. 4
Microtubule disrupting agents does not affect the DNA damage-induced IκB-α degradation and nuclear translocation of NF-κB subunit p65. HeLa cells were treated with Adr (10 µg/mL) or Cpt (100 µM) for various times as indicated in the presence or absence of Col (10 µM), Vin (1 µM) and Noc (0.5 µM). (A, B) Total cell extracts were prepared and applied to SDS-PAGE for immunoblotting with anti-IκB-α and anti-IKK-β antibodies. (C, D) Nuclear extracts were obtained as described Fig.1B, and the nuclear translocation of NF-κB subunit p65 was analyzed by immunoblotting with anti-p65 and anti-p84N5 antibodies from nuclear extracts (50 µg) from each sample.

Fig. 5
Microtubule disrupting agents sensitizes DNA damage-induced apoptotic cell death. (A) HeLa cells were treated with Adr (10 µg/mL) for various times as indicated in the presence or absence of pan-caspase inhibitor z-VAD-FMK (20 µM) and cell extracts were analyzed by immunoblotting with antibodies against casapse-9, -3 and PARP. As a protein loading control, the same amounts of extracts were analyzes by immunoblotting with anti-actin antibody. The asterisks indicate cleaved products of casapse-3 and PARP upon Adr treatment. (B) Wild-type and p65-/- MEF cells were treated with various concentrations of Adr as indicated. 12 hr after treatment, the percentage of cell death was determined by trypan blue exclusion assay as described under Materials and Methods. The results represent the mean values of at least three independent experiments. *, P<0.05, compared with Adr-treated wild-type MEF cells. (C) The expression levels of p65 and IKK-β in wild-type and p65-/- MEF cells. The equal amount of cell extract from each cells was analyzed by immunoblotting with antibodies against p65, IKK-β and actin. (D) HeLa cells were pretreated with the NF-κB specific inhibitors, 5 µM Bay-11 or 1 µM TPCA-1 for 30 min and then treated with 10 µg/mL Adr for 12 hr. Cell death was quantified as described in (B), and each column shows mean±S.E. of at least three independent experiments. *, P<0.05, compared with Adr-treated group. (E) HeLa cells were treated with Adr (10 µg/mL) for 15 hr in the presence or absence of Col (10 µM), Vin (1 µM) and Noc (0.5 µM), or z-VAD-FMK (20 µM). Then, cells were visualized with a normal light microscope with an inverted microscope. (F) HeLa cells were treated with Adr (10 µg/mL) for various times as indicated in the presence or absence of Col (10 µM), Vin (1 µM) and Noc (0.5 µM). Cell death was quantified as described in (B), and each column shows mean±S.E. of at least three independent experiments. (G) HeLa cells were treated as described in (C). Cell extracts form each sample were analyzed by SDS-PAGE followed by immunoblotting with antibodies against casapse-9, -3, PARP and actin. The asterisks indicate cleaved products of casapse-3 and PARP.
References
1. Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005. 5:749–759.
2. Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010. 14:45–55.
3. Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995. 267:1485–1488.

4. Hur GM, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S, Liu ZG. The death domain kinase RIP has an essential role in DNA damage-induced NF-kappaB activation. Genes Dev. 2003. 17:873–882.
5. Waddick KG, Uckun FM. Innovative treatment programs against cancer: II. Nuclear factor-kappaB (NF-kappaB) as a molecular target. Biochem Pharmacol. 1999. 57:9–17.
6. Daroczi B, Kari G, Ren Q, Dicker AP, Rodeck U. Nuclear factor kappaB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther. 2009. 8:2625–2634.
7. Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010. 1805:167–180.
8. Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA. 2004. 101:2452–2457.
9. Starenki D, Namba H, Saenko V, Ohtsuru A, Yamashita S. Inhibition of nuclear factor-kappaB cascade potentiates the effect of a combination treatment of anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2004. 89:410–418.
10. Ahmed KM, Li JJ. ATM-NF-kappaB connection as a target for tumor radiosensitization. Curr Cancer Drug Targets. 2007. 7:335–342.
11. Fan Y, Dutta J, Gupta N, Fan G, Gélinas C. Regulation of programmed cell death by NF-kappaB and its role in tumorigenesis and therapy. Adv Exp Med Biol. 2008. 615:223–250.
12. Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, Evans CP, Zhou Q, Gao AC. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010. 70:3309–3319.
13. Puvvada SD, Funkhouser WK, Greene K, Deal A, Chu H, Baldwin AS, Tepper JE, O'Neil BH. NF-kB and Bcl-3 activation are prognostic in metastatic colorectal cancer. Oncology. 2010. 78:181–188.
14. Rosette C, Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappaB. J Cell Biol. 1995. 128:1111–1119.
15. Das KC, White CW. Activation of NF-kappaB by antineoplastic agents. Role of protein kinase C. J Biol Chem. 1997. 272:14914–14920.
16. Mackenzie GG, Keen CL, Oteiza PI. Microtubules are required for NF-kappaB nuclear translocation in neuroblastoma IMR-32 cells: modulation by zinc. J Neurochem. 2006. 99:402–415.
17. Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C. Transcription factor NF-kappaB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS One. 2007. 2:e589.
18. Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappaB activation by ultraviolet light not dependent on a nuclear signal. Science. 1993. 261:1442–1445.
19. Doostzadeh-Cizeron J, Terry NH, Goodrich DW. The nuclear death domain protein p84N5 activates a G2/M cell cycle checkpoint prior to the onset of apoptosis. J Biol Chem. 2001. 276:1127–1132.

20. Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage (Review). Oncol Rep. 2005. 14:595–599.

21. Berenson JR, Ma HM, Vescio R. The role of nuclear factor-kappaB in the biology and treatment of multiple myeloma. Semin Oncol. 2001. 28:626–633.
22. Um JH, Kang CD, Lee BG, Kim DW, Chung BS, Kim SH. Increased and correlated nuclear factor-kappa B and Ku autoantigen activities are associated with development of multidrug resistance. Oncogene. 2001. 20:6048–6056.

23. Meulmeester E, Jochemsen AG. p53: a guide to apoptosis. Curr Cancer Drug Targets. 2008. 8:87–97.

24. Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009. 458:1127–1130.

25. Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001. 29:684–688.

27. el-Deiry WS. Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr Opin Oncol. 1997. 9:79–87.

28. Pérez-Tomás R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006. 13:1859–1876.
29. Németh ZH, Deitch EA, Davidson MT, Szabó C, Vizi ES, Haskó G. Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J Cell Physiol. 2004. 200:71–81.
30. Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C. TNF-alpha mediated transport of NF-kappaB to the nucleus is independent of the cytoskeleton-based transport system in non-neuronal cells. Eur J Cell Biol. 2006. 85:529–536.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download