Abstract
The incidence of lymphangiomas in the gastrointestinal tract is low, particularly in the colon and rectum, and most cases are solitary. Lymphangiomatosis of the colon are encountered infrequently with only one report in the English literature, and polypectomy was performed for the diagnosis in that case report. However, trends in the diagnosis of lymphangiomatosis of colon have been changing since the development of endoscopic ultrasonography (EUS), and this case is the first in that lymphangiomatosis of the colon was diagnosed without invasive procedures. Here we describe the case of 31-yr-old woman with lymphangiomatosis of the colon with numerous polyposis-like appearing lesions diagnosed by endoscopic ultrasonography and a colonoscopy.
With the widespread use of colonoscopy, lymphangiomas of the colon have increasingly been identified and reported. Most lymphangiomas of the colon are solitary. The presence of lymphangiomatosis of the colon with numerous polyposis-like appearing lesions is extremely rare (1). Here we report a case of lymphangiomatosis of the colon diagnosed by endoscopic ultrasonography (EUS) and a colonoscopy.
A 31-yr-old woman underwent barium enema examination as part of a medical checkup. She had been healthy without specific complaints and had no significant past medical or family history. The patient was 161 cm in height and weighed 44 kg; she appeared well and the physical examination showed no abnormalities. The complete blood cell count and the biochemical analysis of the blood for hepatic and renal function, urine analysis, and fecal occult blood test were all within normal limits. The tumor markers for CEA and CA 19-9 were also normal. No lesions were observed on the upper endoscopy and the abdominal ultrasound.
On the air contrast barium enema, multiple thumbprint-like lesions ranging from 7 to 20 mm in diameter were observed from the cecum to the hepatic flexure, mainly in the ascending colon (Fig. 1). The colonoscopy revealed clusters of round submucosal tumors with a gentle slope and smooth surface from the cecum to the hepatic flexure (Fig. 2). The color and surface characteristics of the lesions did not differ from those of the surrounding normal mucosa; however, they were noted to be pale to transparent. The lesions had a positive cushion (pillow) sign, and there were no ulcerations or erosions.
The EUS was performed with a radial scanning catheter probe (12 MHz, Fujinon Inc., Saitama, Japan) that showed each of the submucosal lesions as an echo-free cyst, with a clear border, in the submucosal layer (Fig. 3A). The inner structure was uniform and some of the cysts had septal walls (Fig. 3B). Some of the lesions were punctured and aspirated, and a transparent yellowish, serous liquid resembling lymphatic fluid was collected. The submucosal lesions easily caved in during aspiration. The endoscopic biopsy revealed a submucosal cyst with occasional multinucleated cells; however, there were no fat or blood cell components (Fig. 4A). The D2-40 immunostaining, which is a specific lymphatic endothelial marker, showed positive reactivity for lining endothelial cells of the lymphatic spaces (Fig. 4B).
Based on the above colonoscopy and EUS findings, the diagnosis of lymphangiomatosis of the colon was made. Further therapy was not provided, because these lesions are benign and the patient had no symptoms associated with them.
Lymphangiomas are composed of multiple dilated lymphatic channels lined by endothelial cells. Most lymphangiomas of the colon are reported as solitary. There are very rare reports of cases with multiple lymphangiomas (1-3). In our case, a number of aggregated tumors with a polyposis-like appearance were distributed only in the colon, without tumor lesions or abnormal findings in other organs. There is only one prior case report in the English literature with lymphangiomatosis of the colon similar to this case (1). Although the mechanism underlying lymphangioma formation is unknown, they are generally regarded as developmental malformations of the lymphatic tissue; obstruction or agenesis of lymphatic tissue causes abnormal dilatation and mass like proliferation of lymphatic channels that finally form a cystic mass. Clinical signs and symptoms of lymphangioms of the colon are varied and non-specific (4). Our patient had no complaints attributable to the lesions.
In the present case, multiple thumbprint-like lesions were observed on the air contrast barium enema, and cystic submucosal tumors with a positive cushion sign were observed on the colonoscopy. The usefulness of EUS, in the qualitative assessment of submucosal tumors of the colon, is well recognized (5-7). There are several reports that confirm the efficacy of EUS for the diagnosis of lymphangioma of the colon (8-10). The characteristic EUS features of the lymphangioma are localization to the submucosal layer, a homogenous echo pattern and multilocular cystic features that are echo-free or contain low-echo septal structures, as in our case. A definitive diagnosis of the submucosal lesions can be obtained only by complete extirpation that includes the submucosal layer of the affected colon, and polypectomy was performed for diagnosis in the previous case report of colonic lymphangiomatosis (1). However, trends in the diagnosis of lymphangiomatosis of colon have been changing since the development of EUS, and the diagnosis of lymphangiomatosis of the colon may be made if the typical characteristics can be demonstrated on a colonoscopy and EUS (10). In the present case, transparent yellowish, serous liquid resembling lymphatic fluid was obtained by the EUS-guided fine needle aspiration and the biopsy also provided additional diagnostic information
Endoscopic biopsies are usually not deep enough to permit evaluation of the submucosa and contained cysts; therefore, the biopsy was not diagnostic in our case. However, some lining endothelial cells of the lymphatic spaces were identified by specific lymphatic endothelial marker and occasional multinucleated cells were noted. In lymphangiomas, some of the lining epithelial cells may be transformed into cuboidal cells or multinucleated giant cells as in our case (1). The multinucleated giant cells may be the result of a poorly defined cell membrane or histiocytic changes in response to lymph fluid released by partial rupture of the lymphangioma wall (1). In this regard, the results of the endoscopic biopsy in our case may provide additional information for diagnosis.
In the past, lymphangiomas of the colon were treated by surgical resection due to the unclear nature of the lesion (11, 12). However, aggressive surgery should be avoided in these cases because it is now known that these lesions are benign. Kochman et al. (8) suggested that asymptomatic lymphangiomas should probably be left alone. Therefore, we elected not to treat our asymptomatic patient with invasive procedures such as endoscopic or surgical resection.
In conclusion, trends in the diagnosis of lymphangiomatosis of colon have been changing since the development of EUS, and this case is the first in that lymphangiomatosis of the colon was diagnosed without invasive procedures. If the typical colonoscopy and EUS findings are observed, the diagnosis of lymphangiomatosis of the colon may be made without invasive diagnostic procedures.
Figures and Tables
Fig. 1
Air contrast barium enema showed multiple thumbprint-like lesions (arrows), ranging from 7 to 20 mm in diameter, mainly in the ascending colon.
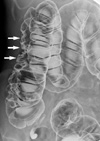
Fig. 2
Endoscopic views of a cluster of elevated lesions, with a smooth surface and gentle slope, in the ascending colon. (A, B) The overlying mucosa was intact and appeared thin, and the lesion was soft and compressible.
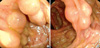
Fig. 3
EUS images of the colon, obtained with a catheter EUS probe (frequency 12 MHz). (A) The EUS image depicting elevated lesions as echo-free cysts (arrowheads) in the submucosal layer. (B) Some submucosal cysts had septal walls (arrows).
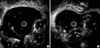
Fig. 4
Microscopic findings. (A) Endoscopic biopsy revealed submucosal cyst with occasional multinucleated cells (inset), however, there were no fat or blood cell components (H&E, ×20; inset: H&E, ×400). (B) D2-40 immunostaining showed positive reactivity (arrows) for lining endothelial cells of lymphatic spaces (Polymer method, ×200).

References
1. Watanabe T, Kato K, Sugitani M, Hasunuma O, Sawada T, Hoshino N, Kaneda N, Kawamura F, Arakawa Y, Hirota T. A case of multiple lymphangiomas of the colon suggesting colonic lymphangiomatosis. Gastrointest Endosc. 2000. 52:781–784.

2. Yoshitoshi Y, Oda T, Utsumi Y, Kaneko E, Yamashita K. Case of lymphangioma of the ascending colon. Nippon Rinsho. 1976. 23:2264–2267.
3. Amaike H, Akioka K, Fujino H, Tanimukai S, Ameno H, Ann T, Nishimoto T, Ikeda E, Muto F, Kurioka H, Hashimoto K, Oouchi T, Tanake K, Harada Y, Ishimine G. A case report of multiple lymphangioma of the colon. Jpn J Surg. 1990. 23:1947–1951.

4. Young TH, Ho AS, Tang HS, Hsu CT, Lee HS, Chao YC. Cystic lymphangioma of the transverse colon: report of a case and review of the literature. Abdom Imaging. 1996. 21:415–417.

5. Shimizu S, Tada M, Kawai K. Use of endoscopic ultrasonography for the diagnosis of colorectal tumors. Endoscopy. 1990. 22:31–34.

6. Kawamoto K, Ueyama T, Iwashita I, Utsunomiya T, Honda H, Onitsuka H, Haraguchi Y, Kojima N, Takano H, Masuda K. Colonic submucosal tumors: comparison of endoscopic US and target air enema CT with barium enema study and colonoscopy. Radiology. 1994. 192:697–702.
7. Fujimura Y, Nishishita C, Iida M, Kajihara Y. Lymphangioma of the colon diagnosed with an endoscopic ultrasound probe and dynamic CT. Gastrointest Endosc. 1995. 41:252–254.

8. Kochman M, Wiersema M, Hawes R, Canal D, Wiersema L. Preoperative diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound. Gastrointest Endosc. 1997. 45:204–206.

9. Hizawa K, Aoyagi K, Kurahara K, Suekane H, Kuwano Y, Nakamura S, Fujishima M. Gastrointestinal lymphangioma: endosonographic demonstration and endoscopic removal. Gastrointest Endosc. 1996. 43:620–624.

10. Irisawa A, Bhutani MS. Cystic lymphangioma of the colon: endosonographic diagnosis with through-the-scope catheter miniprobe and determination of further management. Report of a case. Dis Colon Rectum. 2001. 44:1040–1042.
11. Kuroda Y, Katoh H, Ohsato K. Cystic lymphangioma of the colon: report of a case and review of the literature. Dis Colon Rectum. 1984. 27:679–682.
12. Kuramoto S, Sakai S, Tsuda K, Kaminishi M, Ihara O, Oohara T, Jinbo S, Murakami T. Lymphangioma of the large intestine. Report of a case. Dis Colon Rectum. 1988. 31:900–905.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download