Abstract
Despite the rarity in incidence and prevalence, gastrointestinal stromal tumor (GIST) has emerged as a distinct pathogenetic entity. And the clinical management of GIST has been evolving very rapidly due to the recent recognition of its oncogenic signal transduction pathway and the introduction of new molecular-targeted therapy. Successful management of GIST requires a multidisciplinary approach firmly based on accurate histopathologic diagnosis. However, there was no standardized guideline for the management of Korean GIST patients. In 2007, the Korean GIST study group (KGSG) published the first guideline for optimal diagnosis and treatment of GIST in Korea. As the second version of the guideline, we herein have updated recent clinical recommendations and reflected changes in diagnosis, surgical and medical treatments for more optimal clinical practice for GIST in Korea. We hope the guideline can be of help in enhancing the quality of diagnosis by members of the Korean associate of physicians involving in GIST patients's care and subsequently in achieving optimal efficacy of treatment.
Gastrointestinal stromal tumor (GIST) is relatively rare neoplasm occurring in the gastrointestinal tract, omentum, or mesentery, but is the most common among sarcoma of the gastrointestinal tract and accounts for 5% of all sarcoma. In general, only complete resection can lead to cure, but recurrence in the liver and peritoneum is common after surgery, and unresectable or recurrent tumor does not respond to conventional cytotoxic chemotherapy and therefore, its prognosis has been very poor. However, the identification of signal transduction pathway associated with the development of GISTs and the use of so-called molecular targeted therapy with imatinib (Glivec, Novartis Korea, Seoul, Korea) have yielded remarkable achievement. In addition, imatinib has been shown not only the prolongation of survival time but also the great effectiveness on quality of life with very mild side effects compared with conventional cytotoxic chemotherapy. Based on these results, imatinib is used as first-line therapy in metastatic GISTs, and furthermore, neoadjuvant and adjuvant treatments with imatinib are being investigated.
In the western countries, histopathologic criteria and molecular pathologic mechanism of GIST have recently standardized and the guidelines for this entity have been published by the National Comprehensive Cancer Network (NCCN) (1) and the European Society of Medical Oncology (ESMO) (2). And in Japan and Australia, the guidelines appropriate for clinical practice in each country have been also published (3, 4)
However, in Korea, no standardized guidelines for diagnosis or treatment of GISTs are available. As a result, diagnosis is not consistent between institutions and in addition to the problem associated with diagnosis, optimal treatments are sometimes not provided because of numerous uncertainties related to treatment and a lack of treatment guidelines. To recognize and to find ways to solve these problems, pathologists, surgeons, gastroenterologists, diagnostic radiologists, and medical oncologists organized a multidisciplinary study group called the Korean GIST Study Group (KGSG) in December in 2006. We made the first guideline in 2007 for diagnosis and treatment of GISTs that is suitable for clinical practice in Korea (5). In this second version of the guideline, we sought to update changes in the topics and reflect modified and added recommendations. Expert panel members of the KGSG thoroughly reviewed the relevant literature including the European Society of Medical Oncology and National Comprehensive Cancer Network guidelines and shared their experience and opinions to make a consensus on twenty topics related to pathologic diagnosis, surgical and medical treatment of GIST. The consensus was presented as the basis for a guideline of diagnosis and treatment for patients with GIST that would be used to facilitate the optimal clinical practice in Korea.
GIST is the most common mesenchymal tumor of the gastrointestinal tract (6). GISTs arise from the interstitial cells of Cajal or their common stem cell (7). GISTs range in size from tiny tumors discovered incidentally during tests for other diseases, measuring less than 1 cm to very large lesions measuring upwards of 35 cm (median 5 cm) (8). Irrespective of tumor size, GISTs share morphologic features and immunoreactivity for KIT and contain an oncogenic mutation in the KIT (80-85%) or platelet-derived growth factor receptor (PDGFRA, 5-7%) genes (9). GISTs can arise in any portion of the gastrointestinal tract, but usually occur in the stomach (60%) or the small intestine (30%) (10, 11).
On gross examination, GIST is a well circumscribed, fleshy, pink, or tan-white mass. Large tumors frequently show hemorrhage, necrosis, and cystic degeneration. Microscopically, GISTs can be divided into three different histologic subgroups. Spindle cell GISTs (70%) are composed of cells with palely eosinophilic, fibrillary cytoplasm, ovoid uniform nuclei, and ill-defined cell borders, often with a somewhat syncytial appearance, arranged in short fascicles or whorls (Fig. 1). Epithelioid GISTs (20%) are composed of rounded cells with eosinophilic to clear cytoplasm arranged in sheets and nests (Fig. 2). The final group shows mixed spindle and epithelioid cells (10%). The frequency of these histological types varies according to location. GISTs of the stomach mostly fall into one of 4 spindle cell subtypes of sclerosing, palisading-vacuolated, hypercellular, and sarcomatous or one of 4 epitheloid subtypes of sclerosing epitheloid variant, dyscohesive epithelioid, hypercellular, and sarcomatous (12). GISTs of the small intestine have great amounts of skeinoid fiber, and are most likely to become malignant if epitheloid type or mixed type is present. Many of GISTs in the large intestine are spindle cell type. GISTs developed in the omentum are similar to histological types of the stomach whereas GISTs of the mesentery are similar to histological findings of the small intestine. The diagnosis of GISTs is mainly based on clinical and histological findings, but immunohistochemical staining is needed to confirm diagnosis (13).
The most important immunohistochemical staining in the diagnosis of GISTs is c-kit (CD 117), and other several antibodies may be helpful in the diagnosis and differential diagnosis. Approximately ~95% of cases are positive for c-kit protein (Figs. 1, 2). c-kit negative GISTs account for ~5% of cases and can cause diagnostic difficulties, but given the rather limited choice in mesenchymal diagnostic considerations at these sites, they can often be diagnosed by excluding other potential mimics by immunohistochemical characterization (14). c-kit staining with polyclonal anti-c-kit antibody is mandatory for diagnosis. Extreme caution should always be taken to avoid false-positive or false-negative c-kit staining results by carefully observing positive control (mast cells or interstitial cells of Cajal) and negative control (smooth muscle cells or endothelial cells). Because c-kit may also be positive for other soft tissue tumors, interpretation of c-kit based on H&E findings is necessary (Table 1).
CD34 is positive in 60-80% of GISTs, and the frequency of CD34 positivity depends on location of GISTs. The frequency of positive CD34 is high in GISTs of the esophagus and colon (95%), but relatively low in the small bowel and extra-gastrointestinal sites. In the small intestine, CD34 is positive in 50% of cases while c-kit is positive in almost 100% of cases. However, GISTs of the colon can readily be misdiagnosed as other soft tissue tumors such as inflammatory fibroid polyp or inflammatory myofibroblastic tumor, which is attributable to the rare occurrence of GISTs in the colon and greater incidence of negative or focal staining of c-kit in GISTs of the colon relative to other organs. Thus, negativity of c-kit staining does not exclude a possibility of GIST, and every effort should be made to obtain the diagnosis of GISTs through proper differential diagnoses. Protein kinase C (PKC)-theta staining is positive in approximately 90% of GISTs. The quality of PKC-theta staining must be managed by observing ganglion cells of the intermyenteric plexus as an internal positive control and smooth muscle or blood vessel as a negative control. When the staining is properly performed, it can serve as an important adjunct tool in the diagnosis of c-kit negative GISTs, particularly developed in the stomach and extragastrointestinal locations (15). H-caldesmon is positive in 60-80% of GISTs, which may be helpful in the diagnosis of c-kit negative GISTs. Smooth muscle actin is positive in 30-40% of GISTs, and the frequency of the positive staining is high especially in the small bowel. S-100 and desmin is positive in 5% and 1-2% of GISTs, respectively. A recently developed antibody against DOG1 (discovered on GIST) was reported to be superior in sensitivity and specificity to c-kit and CD34. However, c-kit negative GISTs express DOG1 in only 36% of cases, limiting its use in this setting (16). Fig. 3 shows the algorithm of diagnosis in GISTs based on the immunohistochemical staining results.
In addition to the gastrointestinal tract, GISTs are also found in extragastrointestinal sites, although rare. Caution should be taken because histological and immunohistochemical findings of EGISTs are different from those of GISTs and consequently, it may be very difficult to make a diagnosis.
In GISTs, c-kit is negative in ~5% of cases. These c-kit negative GISTs are common in the stomach and omentum/mesentery. In such cases, examining other immunohistochemical markers (PKC-theta, CD34, SMA or DOG1) and mutation analyses may be useful in diagnosis. Among c-kit negative GISTs, 75% are positive for PKC-theta, 44% for CD34, 40% for SMA, and 36% for DOG1 (14, 15, 17-19).
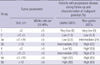
Morphologic risk assessment in GISTs provides the basis for clinical management and optimal patient care. The vast majority of studies of GISTs suggest that the two most important prognostic features to assess the risk of aggressive behavior in a primary localized GIST are mitotic activity and tumor size. These two features were the foundation of the consensus approach for risk assessment in GISTs published by Fletcher and colleagues in 2002 (13). Subsequent data collected by Miettinen and colleagues (20), analyzing large series of GISTs, confirmed that tumor size and mitotic activity are essential prognostic parameters; they proposed additional new parameter, location of tumor, in the evaluation of the clinical behavior of localized GISTs, and KGSG adopted this risk stratification with slight modification (Table 2) (12).
It has been suggested that one block per centimeter should be examined histologically. The pathology report should include the size of the tumor, mitosis (per 50 high powered fields [HPF]), location, resection margin status, and the presence or absence of metastases. Presence of metastasis or perforation during operation leads to a diagnosis of malignant GIST. Pathologic report may include histological type including the degree of cellularity and atypia, presence of necrosis or cystic change, invasion into mucosa or adjacent structures.
At present, mutation analysis is not required for the diagnosis of GIST when tumors have a typical histology and immunohistochemical staining pattern. However, because the presence and location of mutations in KIT or PDGFRA can have implications for prognosis and management in patients with advanced disease, mutation analysis should be considered at the time of diagnosis. Mutational analysis for KIT exons 9, 11, 13, and 17 or PDGFRA exons 12, 14, and 18 can be performed with unstained slides from formalin-fixed paraffin-embedded tissue or fresh frozen tissue.
The main treatment of resectable localized GIST is surgery. The goal is complete resection without residual tumor cells (R0).
The initial diagnosis is generally made by endoscopy, endoscopic ultrasound, gastrography, or computed tomography (CT) of the abdomen due to difficulty with obtaining adequate tissues. It should be confirmed by pathologic histological findings after resection. Preoperative histological diagnosis is feasible, but it may be difficult to interpret definitively (21-24). Imaging tests to detect metastasis include chest radiography (or chest CT), triphasic CT of the abdomen and pelvis, and/or magnetic resonance imaging (MRI) if necessary (25). Positron emission tomography (PET) may be performed when evidence of metastasis may be equivocal or for clinical trials (21).
There is no consensus regarding the need of endoscopic ultrasound biopsy or percutaneous biopsy for preoperative diagnosis. The important part of histological diagnosis is not to cause tumor seeding during biopsy. Therefore, unless multiple metastases are present, excisional biopsy with laparotomy is suggested (21, 26). If diagnosis is unknown at the time of resection, post-operative frozen tissue examination must be performed in order to elucidate the treatment strategy for GIST as treatment varies for an adenocarcinoma or lymphoma. Biopsy is necessary when planning neoadjuvant therapy.
Due to the high potential for malignancy of GIST, resection should be the first-line treatment (21, 27). We strongly recommend resection for tumors larger than 2 cm or growing tumors (21). Smaller tumors (<2 cm) confer a lower potential for malignancy and may be observed. However, small tumor size does not exclude the potential for malignant transformation. Therefore, patients should be informed about the possibility of malignancy.
The main objectives of surgical treatment are to acquire negative margins and to resect without causing tumor rupture. In case of inadvertent tumor infiltration into the surrounding organs, a complete en bloc resection with negative margins should be performed (21, 26, 27) regardless of size. Therefore, even tumors are small, endoscopic shell-out procedure or enucleation should be avoided if GIST is suspected. In many cases, wedge resection of gastric GIST and segmental resection of small bowel GIST are appropriate treatments. Subtotal or total gastrectomy may be performed based on size and location. We recommend en bloc resection for omental or mesenteric GIST. Adjacent organs adherent to tumor should also be completely resected en bloc to avoid tumor rupture or intraabdominal seeding (26).
Laparoscopic resection is feasible if intraabdominal tumor rupture or seeding is unlikely. Laparoscopic resection should follow principles of oncologic surgery. Generally, it is reserved for small, favorably located gastric GISTs (28-31). Intra-operative endoscopy or laparoscopic ultrasound may be used to assist in laparoscopic resection if needed.
Unlike adenocarcinoma, GIST rarely metastasizes to local regional lymph nodes. Therefore, lymphadenectomy is warranted only if metastasis is suspected, i.e. enlarged lymph nodes.
For patients in the high- or intermediate-risk group, we recommend follow-up with CT of the abdomen and pelvis every 3 to 4 months for the first 3 yr after surgery and then every 6 months until 5 yr; then annually thereafter (21, 26). For patients in the low or very low risk, we recommend follow-up with CT every 6 months for 5 yr. Ultrasonography may replace CT once a year (21, 26).
The role of PET for this purpose is not established, and clinical studies on the role of PET are ongoing. Most recurrences occur within 2 yr after surgery and the liver and peritoneum are the most common sites of recurrence (32). Due to the high incidence of gastric cancer in Korea, the National Cancer Screening program recommends biennial stomach-cancer screening for men and women older than 40 yr with endoscopy or upper-gastrointestinal series.
Medical treatment with imatinib alone usually does not result in complete response in metastatic GIST. And, responses are not usually maintained indefinitely. It is now well recognized that clones of tumor cells resistant to imatinib develop continuously over time after start of imatinib. Therefore, in cases where partial or stable responses are shown after adequate duration of imatinib therapy (usually 4 to 12 months of treatment), complete surgical resection of residual tumor may be considered to reduce the risk of development of resistant clones by eradicating the residual viable tumor cells. Several retrospective studies suggested that resection of residual lesions could prolong progression-free survival if it is done during the tumors are under control with imatinib (either in PR or SD) (33, 34). It is also emphasized that imatinib should be continued after resection (21). However, the role of resection of residual tumors after imatinib therapy has not been established, and several phase III clinical trials to investigate the role of surgical resection in this setting are ongoing or planned worldwide. Hepatic or peritoneal metastases may be locally treated with radiofrequency ablation (RFA) or chemoembolization. Importantly, management of GIST should be coordinated by a multidisciplinary team of experienced medical and surgical specialists.
Adjuvant treatment with imatinib is given to enhance the possibility of cure by eradicating microscopic lesions that might still be present after complete resection of visual tumors. Given the great efficacy of imatinib on metastatic or recurrent GIST, imatinib appears to have a sufficient potential as adjuvant treatment.
In recently published ACOSOG Z9001 study of patients with tumor diameter ≥3 cm who received imatinib for 1 yr following complete resection, imatinib demonstrated a significant increase in recurrence-free survival (RFS) compared with placebo although improvement in overall survival (OS) not observed (35). The benefit in RFS appears to be related to tumor size, with the most marked improvement in patients who had large tumor (≥10 cm) in the study.
A Korean phase II study, recently presented in an abstract, evaluated the efficacy of 2-yr imatinib adjuvant therapy in patients who were at high risk of recurrence (tumor size ≥5 cm and mitotic index ≥5/50 HPF, tumor size ≥10 cm, or mitotic index ≥10/50 HPF) based on the NIH risk criteria and had KIT exon 11 mutation which had been recognized as an independent poor risk factor of recurrence in a Korean retrospective study (36, 37). The recurrence-free survival of these patients looked much improved compared with historic data.
These results strongly suggest that adjuvant imatinib therapy should be recommended to reduce the risk of recurrence after curative resection of localized GISTs. However, there are several issues to solve with regard to adjuvant treatment with imatinib. In the ACOSOG Z9001 trial, rapid disease recurrence was observed after stopping 1 yr of imatinib adjuvant treatment. The study did not demonstrate a benefit of adjuvant imatinib in terms of overall survival. Adjuvant treatment with imatinib may delay recurrence. But, it may not increase the cure rate or eliminate the disease in patients with metastatic GISTs; therefore the majority of them eventually develop resistance to imatinib, leading to treatment failure despite its great efficacy. Although currently it remains yet to be determined whether adjuvant imatinib improves cure rate or just delays recurrence, adjuvant imatinib can be proposed as an option for patients at a substantial risk of relapse. Only tumor size and mitotic count were included in the 2002 NIH Consensus risk classification. More recent risk classifications incorporate primary tumor site and/or tumor rupture in addition to the tumor size and mitotic count. Current consensus of the experts is to recommend adjuvant imatinib for patients at high risk of relapse and not for those at low risk of relapse. But, there is no consensus for the patients at intermediate risk (20, 38). Patients with tumor rupture should be considered as having high likelihood of micrometastasis and treated with imatinib. In addition to the risk assessment, mutational analysis may be helpful for the selection of patients who are not likely to get benefit from the treatment. For example, GIST with D842V mutation in PDGFRα exon 18 is known to be not responsive to imatinib. With the currently available data, it is recommended to use imatinib as an adjuvant treatment for at least one year. And, most of the experts agree that 1 yr of adjuvant imatinib is not long enough for especially patients at high risk. However, optimal duration of adjuvant imatinib remains yet to be determined. Phase III trials comparing one year versus three years and no treatment versus two years of treatment duration are ongoing.
Outside of a clinical trial, neoadjuvant treatment with imatinib is not recommended unless there are clinically significant grounds to improve results of surgery by downsizing tumors with neoadjuvant therapy at the initial diagnosis (21). However, neoadjuvant treatment with imatinib may be considered if R0 resection is not feasible, for the purpose of preserving organ functions in GISTs of the rectum, esophagus, and duodenum, or for complete resection of gastric GISTs accompanied by severe, local infiltration into the pancreas or duodenum (39-41). When such a neoadjuvant treatment is considered, progression and response of tumors before and during the treatment should be assessed very carefully by CT and/or PET scan, and it should be done by an experienced multidisciplinary team. Early assessment of tumor response by CT and/or PET is recommended so that surgery is not delayed in the case of non-responding tumors. Duration of neoadjuvant therapy with imatinib may vary according to response to the treatment, but surgery should be performed after sufficient shrinkage of tumors is observed (typically after 4 to 6 months and within 12 months of imatinib treatment) (21). Mutational analysis might be helpful to exclude GISTs not sensitive to imatinib.
Once an advanced GIST is diagnosed, imatinib should be immediately initiated regardless of the presence or absence of symptoms. It is optimal to administer imatinib in patients with liver metastasis or localized metastasis to the peritoneum because cure is hard to achieve even if tumors are completely resected visually and histologically (32, 42). The concept of adjuvant therapy does not apply in this setting.
The optimal initial dose of imatinib is 400 mg per day. In a large European phase III study that compared 400 mg daily with 800 mg daily, the group with 800 mg daily did not achieve an increased survival rate while side effects were increased (43, 44). However, in subgroup analysis, a clinically significant improvement of progression-free survival was observed in the group with KIT exon 9 mutations that received 800 mg per day. So, high dose imatinib is now recommended in the Western countries as the initial treatment for patients with KIT exon 9 mutant GIST (45). However, it is still unclear if this recommendation is valid for Asian patients as well. In recent two retrospective studies of Korean and Taiwanese patients with GIST, there was no difference in the treatment outcomes according to the KIT genotype with imatinib administered at a dose of 400 mg per day (37), which suggest that 400 mg/day of imatinib might be enough for KIT exon 9 mutant GIST patients in Asia. A larger scale retrospective study is ongoing to address this issue.
Treatment with imatinib should be continued indefinitely unless disease progresses, intolerable adverse events occur, or a patient refuses the treatment. If imatinib is discontinued after tumor response with imatinib is achieved, the disease progresses in most cases. Many patients show responses to the reintroduction of imatinib when the disease progresses following imatinib discontinuation, but imatinib treatment should not be interrupted outside of a clinical trial or unless clinically indicated. Surgical resection of stabilized tumor lesions can be considered optionally with enough discussion and with a multidisciplinary approach.
CT is the most useful tool to date to determine response to treatments. We recommend dynamic or triphasic CT scanning through arteries and veins after contrast enhancement (25). Fluourodeoxyglucose (FDG) PET is highly sensitive in early tumor response, but given its cost and availability, it is not easy to include it in basic imaging tests (46-48). Intervals to determine response may be various according to clinical situations, and tumor response is usually determined every 3 to 4 months after the initial response is confirmed.
Determining tumor response or continuation of treatment solely based on tumor size should be avoided because tumors may enlarge due to intratumoral hemorrhage or myxoid degeneration despite therapeutic effects during the beginning of treatment (25, 49-51). Because GIST, hypervascular neoplasm, exhibits hypoattenuating findings resulting from reduced vascularity, hyaline degeneration, and occasional cystic changes following imatinib treatment, the strict application of the response evaluation criteria in solid tumors (RECIST) or WHO criteria requires caution, and the development of new criteria is warranted. Particularly for liver metastasis, when tumor response is determined solely based on the portal-venous phase CT, small and new lesions with hypodensity may be seen, which are most likely the resulting findings of clear margins secondary to necrosis of preexisting tumors that were present before the treatment with the same radiodensity as the hepatic parenchyma, and they require differential diagnosis (25, 51). Both tumor size and tumor density should be considered for the response evaluation. Improvement of patients' symptoms or degree of a reduction in CT attenuation coefficient (Hounsefield Units, HU) or maximum standardized uptake value (SUVmax) on PET may be used to determine tumor response (25, 49-51).
Recurrence or progression includes appearance of a new lesion at the surgery site, development of a new metastasis, or an increase in tumor size. In some cases, a new intratumoral nodule or an increased solid tissue in hypodense tumors previously responded is identified as recurrence or progression. The patterns of the progression cannot be determined based on the RECIST or WHO criteria, and thus inside and cell walls of each lesion should be examined very carefully (25, 52).
Resistance is classified into primary resistance and secondary resistance. Primary resistance is defined as progression within the first 6 months of imatinib therapy, and most of the cases progress multifocally (21). Secondary resistance is defined as progression after 6 months of the initiation of imatinib therapy, and generally two types of progression are seen (21, 25).
Focal progression: It is called when one or a limited number of multiple lesions exhibits intratumoral nodules or lesions become larger, which results in an increased FDG uptake on PET scan, and the remainder of the lesions are relatively well controlled. Treatment for the focal progression requires multidisciplinary approaches. Local treatment such as resection of localized metastasis to the liver or peritoneum, radiofrequency ablation, as well as chemoembolization can be considered to control the focal progressing lesions. But, systemic treatment should be also continued to control hidden micrometastatic lesions. If local progressing lesion(s) was removed, standard dose of imatinib can be continued. But, if those lesions were not removed, dose escalation of imatinib or switch to sunitinib should be considered. No prospective study on the efficacy of local treatment on focal progression has been conducted. Some studies suggest that a part of patients with focal progression may get benefit from local treatment on focal progression, but others eventually show general progression within short time after local treatment (33, 34, 53).
General progression (multifocal resistance): It is called when the majority of multiple lesions simultaneously progress. The efficacy of local treatment on the multifocal resistance is extremely limited and mostly negative (33). Therefore, local treatment for general progression is not recommended except for palliation of symptoms. Administration of imatinib at increased doses or second-line drug such as sunitinib should be considered.
Increase of imatinib dose during disease progression: When disease progresses at the dose of 400 mg per day, an increase of imatinib to 800 mg per day has been known to show partial responses or control of tumors for a certain period in about 30-40% of patients (43, 54). With the daily dose of 800 mg, adverse events associated with imatinib are not increased except malaise and anemia, but if an intolerable side effect occurs, it may be reduced to 600 mg per day (54). When severe adverse events are expected with direct dose escalation to 800 mg per day, imatinib can be first escalated to 600 mg per day, and then sequentially to 800 mg per day. The median progression-free survival is about 3 months and a 12-month progression-free survival rate is 18-30% with the dose escalation of imatinib (55).
Use of sunitinib during disease progression: Sunitinib is a new tyrosine kinase inhibitor which can confer antitumor activity by both direct antitumor activity and antiangiogenic activity because it can inhibit VEGFR as well as kit or pdgfra (56). Sunitinib was approved for the treatment of patients with advanced GISTs after failure of the 1st line imatinib. A phase III pivotal study results showed that sunitinib at a dose of 50 mg daily with 4 weeks on and 2 weeks off schedule was significantly superior in time to progression over placebo (median 27.3 weeks vs 6.4 weeks, P<0.001) (56). Since progression during the rest period was occasionally observed, a continuous dosing schedule with a lower daily dose (37.5 mg per day) was also developed and proven to be effective and well tolerated, although no randomized trials have been performed to compare the intermittent and the continuous dosing schedule. So, the continuous dosing schedule with a lower daily dose can now be considered as an option on an individualized basis.
Use of conventional cytotoxic chemotherapy during disease progression: No conventional cytotoxic chemotherapy has ever been reported to be effective in GISTs. Thus, we do not recommend the use of such drugs except in clinical studies (21).
Continuous use of imatinib or sunitinib after failure of both imatinib and sunitinib: There is no effective systemic treatment option after failure of both imatinib and sunitinib. And, general oncology principles indicate that the use of the same agent that already failed is not beneficial or recommended. However, in spite of resistance to these agents in a majority of tumor cells in this situation, at least a fraction of tumor cells may remain responsive to these agents. So, it is allowed and recommended in many countries to continue one of these agents to slow down the progression of the disease even after the tumor is determined resistant according to the RECIST criteria. For this indication, imatinib may be preferred to sunitinib and daily 400 mg imatinib may be appropriate. However, this issue remains to be addressed in a well designed clinical trial.
Although several clinical practice guidelines for GIST based on clinical practice of each country have recently been published by the study group of each country (NCCN, ESMO, the Japan Society of Clinical Oncology, and in Australia), there was no standardized guideline for diagnosis or treatment of GISTs in Korea. To solve this problem, pathologists, surgeons, gastroenterologists, diagnostic radiologists, and medical oncologists organized a multidisciplinary study group called the Korean GIST Study Group (KGSG). We made the first guideline in 2007 for diagnosis and treatment of GISTs that is suitable for clinical practice in Korea. This study is the second version of the guideline for Korean GIST patients. Through series of workshops to review and discuss evolving new evidences, we have updated recent clinical recommendations and reflected changes in diagnosis, surgical and medical treatment for more optimal clinical practice for GIST in Korea. We hope the guideline can be of help in enhancing the quality of diagnosis by members of the Korean associate of physicians involving in GIST patients' care and subsequently in achieving optimal efficacy of treatment.
Figures and Tables
Fig. 1
Typical photomicrograph of spindle cell gastrointestinal stromal tumor (A: H&E, ×400) and c-kit stained in the cytoplasm and cytoplasmic membranes (B: Immunohistochemical stain, ×400). Blood vessels within the tumor are negative for c-kit (B).
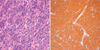
Fig. 2
Typical photomicrograph of epithelioid gastrointestinal stromal tumor (A: H&E, ×400) and c-kit stained in the cytoplasm and cytoplasmic membranes (B: Immunohistochemical stain, ×400).
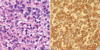
Table 2
Newly proposed risk stratification of primary localized gastrointestinal stromal tumors (12)
References
1. Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009. 20:Suppl 4. 64–67.

2. Zalcberg JR, Desai J, Mann B, Fox S, Goldstein D, Mcarthur G, Clark M, Yip D. Consensus approaches to best practice management of gastrointestinal stromal tumors. Asia Pac J Clin Oncol. 2008. 4:188–198.

3. Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T. GIST Guideline Subcommittee. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008. 13:416–430.

4. Demetri GD, Antonia S, Benjamin RS, Bui MM, Casper ES, Conrad EU III, DeLaney TF, Ganjoo N, Kech R Jr, Heslin MJ, Hutchinson RJ, Kane JM III, Letson GD, McGarry SV, O'Donnell RJ, Paz IB, Pfeifer JD, Pollock RE, Randall RL, Riedel RF, Schupak DD, Schwartz HS, Thomton K, von Mehren M, Wayne JD. National Comprehensive Cancer Network Soft Tissue Sarcoma Panel. National Comprehensive Cancer Network Practice Guidelines in Oncology: Soft Tissue Sarcoma. J Natl Compr Canc Netw. 2010. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
5. Kang YK. Clinical practice guideline for adequate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. J Korean Med Assoc. 2007. 50:830–841.

6. Kang DY, Park CK, Choi JS, Jin SY, Kim HJ, Joo M, Kang MS, Moon WS, Yun KJ, Yu ES, Kang H, Kim KM. Multiple gastrointestinal stromal tumors: Clinicopathologic and genetic analysis of 12 patients. Am J Surg Pathol. 2007. 31:224–232.

8. Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, Fisher GA, Fletcher CD, Gronchi A, Hohenberger P, Hughes M, Joensuu H, Judson I, Le Cesne A, Maki RG, Morse M, Pappo AS, Pisters PW, Raut CP, Reichardt P, Tyler DS, Van den Abbeele AD, von Mehren M, Wayne JD, Zalcberg J. NCCN Task Force. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007. 5:Suppl 2. S1–S29.

9. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003. 21:4342–4349.

10. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. Gastrointestinal Stromal Tumor Committee. Korean Gastrointestinal Pathology Study Group. Gastrointestinal stromal tumors in Koreans: it's incidence and the clinical, pathologic and immunohistochemical findings. J Korean Med Sci. 2005. 20:977–984.

11. Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, Jung JS, Jung ES, Jin SY, Kang DY, Park JB, Park HS, Choi YD, Sung SH, Kim YB, Kim H, Bae YK, Kang M, Chang HJ, Chae YS, Lee HE, Park do Y, Lee YS, Kang YK, Kim HK, Chang HK, Hong SW, Choi YH, Shin O, Gu M, Kim YW, Kim GI, Chang SJ. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010. 25:853–862.

12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006. 130:1466–1478.

13. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002. 33:459–465.

14. Liegl B, Hornick JL, Lazar AJ. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009. 23:49–68.

15. Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. PKCtheta expression in gastrointestinal stromal tumor. Mod Pathol. 2006. 19:1480–1486.
16. Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009. 33:437–446.

17. Lee HE, Kim MA, Lee HS, Lee BL, Kim WH. Characteristics of KIT-negative gastrointestinal stromal tumours and diagnostic utility of protein kinase C theta immunostaining. J Clin Pathol. 2008. 61:722–729.

18. Orosz Z, Tornoczky T, Sapi Z. Gastrointestinal stromal tumors: a clinicopathologic and immunohistochemical study of 136 cases. Pathol Oncol Res. 2005. 11:11–21.

19. Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, Fletcher JA, Fletcher CD. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004. 28:889–894.
20. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006. 23:70–83.

21. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, Le Cesne A, McClure J, Maurel J, Nupponen N, Ray-Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005. 16:566–578.

22. Schwartz SI, Shires GT. Schwartz's principles of surgery. 2005. 8th ed. New York: McGraw-Hill.
23. Townsend CM, Sabiston DC. Sabiston textbook of surgery: The biological basis of modern surgical practice. 2004. 17th ed. Philadelphia: W.B. Saunders Co..
24. Trent JC, Benjamin RS. New developments in gastrointestinal stromal tumor. Curr Opin Oncol. 2006. 18:386–395.

25. Ryu MH, Lee JL, Chang HM, Kim TW, Kang HJ, Sohn HJ, Lee JS, Kang YK. Patterns of progression in gastrointestinal stromal tumor treated with imatinib mesylate. Jpn J Clin Oncol. 2006. 36:17–24.

26. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006. 244:176–184.
27. Iwahashi M, Takifuji K, Ojima T, Nakamura M, Nakamori M, Nakatani Y, Ueda K, Ishida K, Naka T, Ono K, Yamaue H. Surgical management of small gastrointestinal stromal tumors of the stomach. World J Surg. 2006. 30:28–35.

28. Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007. 33:444–447.

29. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006. 243:738–745.

30. Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, Kubota T, Mukai M, Kameyama K, Sugino Y, Kumai K, Kitajima M. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006. 139:484–492.

31. Pister PW, Blay JY. GIST Guidelines: from Evidence to Practice, Evolving Consensus in GIST Management Meeting Monograph. 2007. In : GIST Gloval Opinion Leader Summit; Geneva.
32. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000. 231:51–58.
33. Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Fletcher CD, Demetri GD, Bertagnolli MM. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006. 24:2325–2331.

34. Sym SJ, Ryu MH, Lee JL, Chang HM, Kim TW, Kim HC, Kim KH, Yook JH, Kim BS, Kang YK. Surgical intervention following imatinib treatment in patients with advanced gastrointestinal stromal tumors (GISTs). J Surg Oncol. 2008. 98:27–33.

35. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K. American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009. 373:1097–1104.

36. Kang B, Lee J, Ryu M, Im S, Park S, Kang W, Kim T, Oh D, Jung K, Kang Y. A phase II study of imatinib mesylate as adjuvant treatment for curatively resected high-risk localized gastrointestinal stromal tumors. J Clin Oncol. 2009. 27:Suppl. abstr e21515.

37. Kim TW, Ryu MH, Lee H, Sym SJ, Lee JL, Chang HM, Park YS, Lee KH, Kang WK, Shin DB, Bang YJ, Lee JS, Kang YK. Kinase mutations and efficacy of imatinib in Korean patients with advanced gastrointestinal stromal tumors. Oncologist. 2009. 14:540–547.

38. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008. 39:1411–1419.

39. Bumming P, Andersson J, Meis-Kindblom JM, Klingenstierna H, Engstrom K, Stierner U, Wangberg B, Jansson S, Ahlman H, Kindblom LG, Nilsson B. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: a centre-based study of 17 patients. Br J Cancer. 2003. 89:460–464.

40. Loughrey MB, Mitchell C, Mann GB, Michael M, Waring PM. Gastrointestinal stromal tumour treated with neoadjuvant imatinib. J Clin Pathol. 2005. 58:779–781.

41. Oh JS, Lee JL, Kim MJ, Ryu MH, Chang HM, Kim TW, Jang SJ, Yook JH, Oh ST, Kim BS, Kang YK. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors of stomach: Report of three cases. Cancer Res Treat. 2006. 38:178–183.
42. Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002. 33:466–477.

43. Rankin C, von Mehren M, Blanke C, Benjamin R, Fletcher CD, Bramwell V, Crowley J, Borden E, Demetri GD. Dose effect of imatinib (im) in patients (pts) with metastatic GIST - Phase III Sarcoma Group Study S0033. J Clin Oncol. 2004. 22:abstr 9005.

44. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004. 364:1127–1134.

45. Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Hagemeijer A, Judson I. EORTC Soft Tissue and Bone Sarcoma Group. Italian Sarcoma Group. Australasian GastroIntestinal Trials Group. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006. 42:1093–1103.

46. Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, Bockisch A, Debatin JF, Freudenberg LS. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004. 45:357–365.
47. Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun. 2004. 25:433–438.

48. Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R, Dumez H, Silberman S, Mortelmans L, van Oosterom A. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer. 2003. 39:2012–2020.

49. Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004. 183:1619–1628.
50. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007. 25:1753–1759.

51. Linton KM, Taylor MB, Radford JA. Response evaluation in gastrointestinal stromal tumours treated with imatinib: Misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol. 2006. 79:e40–e44.

52. Desai J, Shankar S, Heinrich MC, Fletcher JA, Fletcher CD, Tuncali K, Silverman SG, Van Den Abbeele AD, Vansonnenberg E, Demetri GD. Clonal evolution of resistance to imatinib (IM) in patients (pts) with gastrointestinal stromal tumor (GIST): Molecular and radiologic evaluation of new lesions. J Clin Oncol. 2004. 22:abstr 3010.

53. Bauer S, Hartmann JT, de Wit M, Lang H, Grabellus F, Antoch G, Niebel W, Erhard J, Ebeling P, Zeth M, Taeger G, Seeber S, Flasshove M, Schütte J. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005. 117:316–325.

54. Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR. EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group. Australasian Gastrointestinal Trials Group. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005. 41:1751–1757.
55. Park I, Ryu MH, Sym SJ, Lee SS, Jang G, Kim TW, Chang HM, Lee JL, Lee H, Kang YK. Dose escalation of imatinib after failure of standard dose in Korean patients with metastatic or unresectable gastrointestinal stromal tumor. Jpn J Clin Oncol. 2009. 39:105–110.

56. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006. 368:1329–1338.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download