Abstract
Acute phlegmonous infection of the gastrointestinal tract is characterized by purulent inflammation of the submucosa and muscular layer with sparing of the mucosa. The authors report a rare case of acute diffuse phlegmonous esophagogastritis, which was well diagnosed based on the typical chest computed tomographic (CT) findings and was successfully treated. A 48-yr-old man presented with left chest pain and dyspnea for three days. Chest radiograph on admission showed mediastinal widening and bilateral pleural effusion. The patient became febrile and the amount of left pleural effusion is increased on follow-up chest radiograph. Left closed thoracostomy was performed with pus drainage. A CT diagnosis of acute phlegmonous esophagogastritis was suggested and a surgery was decided due to worsening of clinical condition of the patient and radiologic findings. Esophageal myotomies were performed and the submucosal layer was filled with thick, cheesy materials. The patient was successfully discharged with no postoperative complication.
Phlegmon is a spreading diffuse inflammatory process associated with the formation of a suppurative exudate or pus. Phlegmonous infection may involve any gastrointestinal tract site, although the stomach is most frequently involved (1). However, phlegmonous involvements of the esophagus, small bowel, or colon are rare (1-3). Phlegmonous infection of the gastrointestinal tract is usually diagnosed at autopsy or surgery. Therefore, strong suspicion and recognition of this disease is a key to the diagnosis and prompt management of patients with acute symptoms. Antibiotic therapy and appropriate surgical drainage are effective treatment modalities for localized disease. However, the role of surgery has been questioned for the diffuse disease form (2). The authors report a rare case of acute diffuse phlegmonous esophagogastritis. In this case, proper radiologic diagnosis with typical chest computed tomography (CT) findings (4,5) enabled appropriate treatment and timely surgical intervention.
A 48-yr-old man presented with left chest pain, abdominal pain, and dyspnea of three days duration. Five days before admission, he had been involved in a minor motorcycle accident, but was asymptomatic for two days. The patient also had a history of chronic alcoholism and uncontrolled diabetes mellitus. He was a nonsmoker.
On admission, he was acutely ill-looking. However, his vital signs were stable; heart rate 70/min, respiration 37/min, blood pressure 110/80 mmHg, and body temperature 36.8℃. A physical examination also revealed no remarkable finding with normal bowel sounds and a soft, flat abdomen with no general or rebound tenderness. Laboratory tests revealed; WBC 3,200/µL, C-reactive protein 31.68 mg/dL, and serum glucose 201 mg/dL, and chest radiography on admission showed mediastinal widening and bilateral pleural effusion (Fig. 1). The patient underwent endoscopy on the admission day to exclude esophageal rupture, and diffuse thickening of mucosal folds with decreased distensibility and an 1 cm sized mucosal ulcer in upper thoracic esophagus were observed with scattered patches of hemorrhage in the gastric mucosa of the body and antrum.
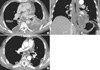
On the evening of first hospital day, the patient was became febrile with a body temperature of 39℃, and thus, empirical treatment with broad spectrum antibiotics was immediately started under the suspicion of empyema or secondary infection. On the second hospital day, the dyspnea worsened and the amount of left pleural effusion increased on chest radiography. Left closed thoracostomy was performed with pus drainage. On the forth hospital day, a contrast-enhanced chest CT scan was performed and showed diffuse and marked circumferential wall thickening of the entire thoracic esophagus, extending to gastric cardia and associated with diffuse intramural low density and a peripheral enhancing rim (Fig. 2A, B). In addition, multiloculated bilateral pleural effusion and mild pleural thickening were evident. A CT diagnosis of acute phlegmonous esophagogastritis was suggested and bilateral open thoracotomies were performed immediately. Pleural fluid analysis revealed exudates and a surgery was decided due to worsening of clinical condition of the patient and radiologic findings.
During surgery, bilateral multiloculated pleural effusions were evacuated through open thoracotomies and the esophagus was freed from adjacent tissue. The adventitial and muscular layers of the esophagus were intact and no perforation was identified. Several separate esophageal myotomies were performed and the submucosal layer was found to have been filled with thick, cheesy materials, which were removed from the mucosa by scraping. Blood and sputum cultures of the patient and a microbiologic examination of pleural fluid demonstrated Klebsiella pneumoniae.
A follow-up chest CT scan performed on the 49th postoperative day showed reduced diffuse esophageal wall thickening and bilateral pleural effusion (Fig. 2C). The patient was successfully discharged on the 73rd postoperative day.
Phlegmonous infection can affect any site of the gastrointestinal tract, although the stomach is most frequently involved (1-3). Involvements of other sites have been rarely reported, but the simultaneous involvement of esophagus and stomach has only been reported in a very limited number (1, 2). Phlegmonous infection usually involves the submucosa and not the mucosa (1-3). Diffuse esophagogastric involvement of phlegmonous inflammation was evident in our case by chest CT and during surgery. In its localized form, an area of acute inflammation in submucosa is noted, usually affecting the gastric antrum. Phlegmonous infection may also present as a mass in the gastric wall (6). The inflammation produced may involve the muscularis mucosa and the serosa, and lead to perforation or even peritonitis (1). In its diffuse form, phlegmonous infection can involve the entire stomach, but it rarely extends beyond the cardia or pylorus (6).
The pathogenesis of acute phlegmonous esophagitis is unclear. Reported predisposing factors (4) include immune suppression, alcoholism, peptic ulcer disease, chronic gastritis or some other gastric mucosal injury, achlorhydria, infection, connective tissue disease, and malignancy. Presumably, these conditions predispose the stomach to infection by eliminating various defense mechanisms, such as, inherent gastric cytoprotection or the bactericidal effect of gastric acid (1, 6). Nevertheless, approximately 50% of reported cases were previously healthy and had no significant anteceding risk factors (6). We consider that uncontrolled diabetes mellitus and a recent history of chest trauma in combination with excessive alcohol consumption played an important role in the development of the disease in our patient.
Histopathologically, the submucosa is thickened and infiltrated by neutrophils and plasma cells with intramural hemorrhage, necrosis, and thrombosis of submucosal blood vessels (1). The most common pathogens are Streptococcus species, Staphylococcus species, Escherichia coli, Haemophilus influenzae, Proteus, and Clostridia (6). Streptococcus accounts for approximately 70 to 75% of cases, and it is also the organism most commonly associated with death caused by phlegmonous gastritis. The causative pathogen of phlegmonous infection in our patient is believed to be Klebsiella pneumoniae, based on positive culture results on blood, sputum and pleural fluid.
Phlegmonous gastritis has rarely been diagnosed before surgery, because it is seldom considered in the differential diagnosis of an acute abdomen (3). Patients usually present with an acute abdomen and septicemia. Other symptoms include nausea, vomiting, hematemesis, hiccups, prostration, and fever (6). On the other hand, when the esophagus is involved, odynophagia, dysphagia, and chest pain are the most common symptoms (1). Conversely, some patients may only have fever and bacteremia.
Phlegmonous infection is usually diagnosed at surgery or at autopsy (3), and because there are no pathognomonic signs or symptoms, phlegmonous gastritis is rarely diagnosed before surgery. Endoscopy of the affected esophagus shows diffuse luminal narrowing with poor distensibility and ulcer-like lesions (3). Endoscopic ultrasonography (EUS) findings in previous case reports were diffuse thickening with hypoechoic lesions in the submucosal layer (1, 6). The endoscopic examination of our patient also revealed similar findings of diffuse mucosal fold thickening, a mucosal ulcer in the upper thoracic esophagus, and scattered hemorrhagic patches in gastric mucosa.
The reported CT findings of acute phlegmonous esophagitis or gastritis include diffuse esophageal and stomach wall thickening with circumferential intramural low attenuation surrounded by a peripheral enhancing rim. The intramural low attenuation represents severe inflammation and abscess localized to the submucosa and muscularis layer (4, 5). Within the thickened wall, air bubbles were seen and probably produced by gas-forming organisms. Contrast-enhanced chest CT in our patient showed findings typical of acute phlegmonous inflammation with simultaneous esophageal and stomach involvement. The radiographic differential diagnoses included a dissecting intramural hematoma and tubular duplication of esophagus and emphysematous esophagitis or gastritis (5, 7). However, the clinical symptom of a dissecting intramural hematoma is chest pain with no evidence of infection or inflammation, and patients with tubular duplication are likely to have no symptoms or signs.
The overall mortality of phlegmonous gastritis in a review of 36 reported cases was 42%, and the mortality rates of the 10 patients that underwent surgical resection as compared with the 26 patients treated conservatively were 20% (2/10) and 50% (13/26), respectively (6). During the last 50 yr, some reports have described patients with phlegmonous gastritis successfully treated with medical therapy alone. Overall, the mortality rate for patients with medially treated localized disease was 17%, whereas that for diffuse disease was 60%. Thus, antibiotic therapy and surgical drainage are effective treatments for acute phlegmonous esophagitis depending on the clinical situation (1, 3, 8). In cases of phlegmonous esophagogastritis, protracted conservative treatment result in surgical resection due to the possibilities of esophageal necrosis, esophageal stricture, gastric mucosal atrophy, and complicated peritonitis (1, 8, 9).
Our patient was initially treated with broad spectrum antibiotics and left closed thoracostomy with empyema drainage. However, surgical intervention was decided upon due to a worsening of his condition, the duration of his clinical symptoms, and radiographic findings, which included a proper CT diagnosis of acute phlegmonous esophagogastritis. We consider that combined medical treatment and timely surgical intervention played an important role in the cure achieved in our patient, who experienced no major post-operative complications.
In conclusion, although acute phlegmonous esophagogastritis is rare and a preoperative diagnosis is difficult, awareness of this disease entity and prompt diagnosis based on typical chest CT findings are major key factors to successful treatment.
Figures and Tables
Fig. 1
Chest radiograph on admission: a chest radiograph obtained on admission shows widening of the mediastinum and the carinal angle with bilateral pleural effusion.

Fig. 2
Initial and post-opearative follow-up chest CT scans. (A, B) Initial chest CT scan with axial (A) and coronal (B) reformation reveals diffuse wall thickening with intramural low density (arrowheads) along the entire length of the thoracic esophagus extending to gastric cardia. Multiloculated bilateral pleural effusion, which proved to be empyema, is also shown. (C) Follow-up chest CT after surgery shows a marked improvement of the diffuse esophageal wall thickening and empyema.
References
1. Hsu CY, Liu JS, Chen DF, Shih CC. Acute diffuse phlegmonous esophagogastritis: report of a survived case. Hepatogastroenterology. 1996. 43:1347–1352.
2. Wakayama T, Watanabe H, Ishizaki Y, Okuyama T, Ogata H, Tanigawa K, Kawahara Y. A case of phlegmonous esophagitis associated with diffuse phlegmonous gastritis. Am J Gastroenterol. 1994. 89:804–806.
3. Lee CR, Lee JH, Choi SJ, Lee DS, Kim WS, Han SR, Chung NW, Park HS, Choi SH. A case of acute phlegmonous esophagitis. Korean J Gastrointest Endosc. 2000. 20:119–123.
4. Yun CH, Cheng SM, Sheu CI, Huang JK. Acute phlegmonous esophagitis: an unusual case (2005: 8b). Eur Radiol. 2005. 15:2380–2381.

5. Jung C, Choi YW, Jeon SC, Chung WS. Acute diffuse phlegmonous esophagogastritis: radiologic diagnosis. AJR Am J Roentgenol. 2003. 180:862–863.

6. Kim GY, Ward J, Henessey B, Peji J, Godell C, Desta H, Arlin S, Tzagournis J, Thomas F. Phlegmonous gastritis: case report and review. Gastrointest Endosc. 2005. 61:168–174.

7. Jung JH, Choi HJ, Yoo J, Kang SJ, Lee KY. Emphysematous gastritis associated with invasive gastric mucormycosis: a case report. J Korean Med Sci. 2007. 22:923–927.

8. Mann NS, Borkar BB, Mann SK. Phlegmonous esophagitis associated with epiphrenic diverticulum. Am J Gastroenterol. 1978. 70:510–513.
9. I H, Park CS, Kim YD. Treatment of phlegmonous esophagitis combined with mediastinitis. Korean J Thorac Cardiovasc Surg. 2007. 40:711–714.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download