Abstract
An 82-yr-old man was presented with fever and cough accompanied by generalized erythematous rash. He had taken mexiletine for 5 months, as he had been diagnosed with dilated cardiomyopathy and ventricular arrhythmia. Laboratory studies showed peripheral blood eosinophilia and elevated liver transaminase levels. Chest radiographs showed multiple nodular consolidations in both lungs. Biopsies of the lung and skin lesions revealed eosinophilic infiltration. After a thorough review of his medication history, mexiletine was suspected as the etiologic agent. After discontinuing the mexiletine and starting oral prednisolone, the patient improved, and the skin and lung lesions disappeared. Subsequently, mexiletine was confirmed as the causative agent based on a positive patch test. Drug-induced hypersensitivity syndrome is a severe adverse reaction to drugs and results from treatment with anticonvulsants, allopurinol, sulfonamides, and many other drugs. Several cases of mexiletine-induced hypersensitivity syndrome have been reported in older Japanese males with manifestation of fever, rash, peripheral blood eosinophilia, liver dysfunction without other organ involvement. Here, we report a case of mexiletine-induced hypersensitivity syndrome which presented as eosinophilic pneumonia in a Korean male.
Drug-induced hypersensitivity syndrome is a severe adverse drug reaction, which often manifests as an erythematous skin eruption, fever, lymphadenopathy, peripheral blood eosinophilia, and visceral organ involvement (1). Many drugs cause hypersensitivity syndrome, including anticonvulsants, sulfonamides, dapsone, allopurinol, minocycline, and gold salts (2). Mexiletine is an antiarrhythmic agent that has been used to treat ventricular tachycardia for more than 30 yr (3). Since the first report by Higa et al. (4). in 1997, a few cases of mexiletine-induced hypersensitivity syndrome have been reported, especially in Japanese males over 45 yr of age (5-8). These cases manifested with fever, rash, peripheral blood eosinophilia, elevation of liver transaminase enzymes without other organ involvement. Here, we present the first case of mexiletine-induced hypersensitivity syndrome with lung involvement, which is also the first case of mexiletine-induced hypersensitivity syndrome in Korea. This case manifested as eosinophilic pneumonia, in addition to fever, a papuloerythematous skin rash, peripheral blood eosinophilia, and liver dysfunction.
An 82-yr-old man was admitted to the hospital with a fever and cough. The patient had been diagnosed with an arrhythmia associated with dilated cardiomyopathy 9 months earlier and had been taking mexiletine for 5 months in addition to furosemide and spironolactone, which was continued since the diagnosis. He developed a fever, cough, and sputum 1 week before admission (Fig. 1). He was a retired pharmacist who lived in downtown Seoul, Korea. On physical examination, variable-sized, fused erythematous macules and plaques covered his entire body, including the trunk and extremities; this rash developed on the day of admission (Fig. 2). His body temperature was 38.3℃, and his respiratory rate was 22 times per min. Auscultation of the lungs disclosed diminished breath sounds throughout both lungs, with crackles at the bases. Laboratory studies showed leukocytosis (13,700/µL) with eosinophilia (3,310/µL; 24.2% of the white blood cells) in the peripheral blood. Liver dysfunction was also detected (aspartate transaminase 193 U/L; alanine transaminase 321 U/L, lactate dehydrogenase 290 U/L). Chest radiographs showed multiple increased opacities with a patchy distribution in both lungs (Fig. 3A). Chest computed tomography (CT) on admission showed multiple nodular consolidations with ground-glass density in both hemithoraxes and multiple mediastinal lymphadenopathy (Fig. 3B). A fine needle aspiration biopsy of a lung lesion was performed and showed eosinophilic infiltration with histiocytes, and granular pneumocytes with an organizing alveolar exudate (Fig. 4A). In addition, a right abdominal skin lesion was biopsied. The dermis showed extravasated red blood cells and moderate perivascular infiltration of lymphocytes and eosinophils (Fig. 4B). After admission, he was diagnosed with type II diabetes mellitus for the first time based on a fasting serum glucose of 183 mg/dL and hemoglobin A1c of 8.6%. The patient was diagnosed with drug-induced hypersensitivity syndrome based on the clinical findings and laboratory evidence. The mexiletine was suspected to cause theses adverse reactions and was withdrawn, while furosemide and spironolactone was continued. For the treatment of severe immune response, prednisolone 0.5 mg/kg was given for 10 days and tapered. Subsequently, his temperature decreased, with resolution of the blood eosinophilia, liver dysfunction, and cutaneous and lung lesions (Fig. 3C). With the resolution of adverse reactions, digoxin was susbstituted for mexiletine to treat arrhythmia.
Four months after resolution of the adverse findings, a patch test was performed to confirm the etiologic diagnosis. Mexiletine (Mexitil®, Boehringer Ingelheim Korea, Seoul, Korea), furosemide (Lasix®, Handok Pharmaceuticals Co., Seoul, Korea), and spironolactone (Aldactone®, Pharmacia Ltd., UK) at concentrations of 0, 1, 2, 5, 10, and 20% in petrolatum was applied at the back of the patient using Finn chambers on Scanpor tape for 48 hr. At 30 min and 48 hr after removal of the tape, the responses were scored according to the International Contact Dermatitis Research Group guidelines (9). Weakly positive reactions were detected with 5, 10, and 20% mexiletine, while ten control subjects showed negative result to the test. Based on clinical course and patch test, we were able to identify mexiletine as the etiologic agent for the development of drug-induced hypersensitivity syndrome. To explore the association of these events with human herpes virus 6 (HHV-6) infections, we performed polymerase chain reaction (PCR) analysis for HHV-6 in the serum of the patient taken at the time of patch test. The PCR analysis did not detect HHV-6 DNA.
Drug-induced hypersensitivity syndrome is the name for the severe adverse reactions to drugs as a result of systemic immune responses and is often called DRESS (drug reaction with eosinophilia and systemic symptoms) (10). Anticonvulsants such as carbamazepine and phenytoin are the most common etiologic agents causing drug-induced hypersensitivity syndrome (11). In addition, allopurinol, sulfonamides, dapsone, and minocycline can cause these reactions. Mexiletine has been reported to cause severe hypersensitivity reactions, including drug-induced hypersensitivity syndrome (or DRESS). Although mexiletine is prescribed worldwide, all previously reported cases of mexiletine-induced hypersensitivity syndrome have been Japanese, suggesting a genetic predisposition (Table 1). The present case is the first report in a non-Japanese population. Despite ethnic differences, the Korean and Japanese populations share much the same genetic background, and the exclusive incidence of mexiletine-induced hypersensitivity syndrome in Far Eastern countries implies that genetic susceptibility underlies its pathogenesis. While genetic variants associated with the development of drug induced hypersensitivity syndrome are not well defined, the human leukocyte antigen HLA-B*1502 is reported to be significantly associated with the development of carbamazepine-induced Stevens-Johnson syndrome (SJS) and toxic epidermal necorlysis (TEN) (12). This genetic association may be ethnicity specific, in that strong association is observed in Han-Chinese, not in Caucasians (13). These finding can explain relatively higher incidence of the event in Han-Chinese than in Caucasians (12, 14). Like carbamazepine-induced SJS and TEN, high incidence of mexiletine-induced hypersensitivity syndrome in Far Eastern countries maybe due to ethnicity specific genetic predisposition. In addition to ethnic characteristics, all reported cases have developed in relatively old males, which suggests a sex and age predilection.
Internal organ involvement is a specific finding of hypersensitivity syndrome, and the specific organ involvement often depends on the etiologic drug (1). Allopurinol-induced reactions frequently involve the kidney, whereas phenytoin and dapsone often cause liver dysfunction. The previous reports of mexiletine-induced hypersensitivity syndrome did not reveal the specific organ involvement, except for liver dysfunction (Table 1). Ours is the first report of lung involvement in mexiletine-induced hypersensitivity syndrome. In this report, we proved eosinophilic lung infiltration with not only imaging studies but also microscopic examination of the lung lesions. In many cases of drug-induced hypersensitivity syndrome, a clinical response develops 3 weeks to 3 months after starting a medication. This was also true for mexiletine-induced cases (Table 1), except for that reported by Yagami et al. (8) and the present case. In these latter two cases, the patients developed clinical manifestations after 5 or 6 months of medication, and patch tests proved mexiletine was the causative agent.
The mechanism for the immunogenicity of these drugs is not clear, although a role of reactive drug metabolites in initiating an immune response via hapten formation has been suggested (15). Recently, the involvement of viral infection in the pathogenesis of drug-induced hypersensitivity syndrome has been explored intensively. Although we did not confirm the viral association in this case, some previous case studies of mexiletine-induced hypersensitivity syndrome have suggested a relationship between human herpes virus 6 and the development of systemic immune responses (6-8).
In conclusion, we report the first case of mexiletine-induced hypersensitivity syndrome with lung eosinophilic infiltrates. This is also the first report of these reactions in a non-Japanese population.
Figures and Tables
Fig. 1
Clinical courses and medications. These graphs illustrate clinical course including fever, rash, lung lesions, peripheral blood eosinophil count (solid line) and serum alanine transaminase (ALT, dashed line) over time. Under the X-axis, the medications used for the treatment of heart failure and systemic steroids are shown.

Fig. 2
Skin lesions on admission. There were variable-sized, occasionally fused, erythematous macules and plaques covering the skin of the entire body.
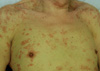
Fig. 3
Imaging examination of the chest. (A) Simple radiographs of the chest on admission show diffuse heterogeneous increased opacities with a patchy distribution in both lungs. (B) Chest CT on admission shows multiple nodular consolidations with ground-glass density in both hemithoraxes and multiple mediastinal lymphadenopathy. (C) Simple radiographs of the chest after discontinuing the mexiletine and then treating the patient with oral prednisolone. The multiple infiltrative lesions disappeared from both lungs.
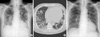
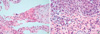
Fig. 4
Microscopic observation of the biopsy specimen. (A) The tissue of the lung lesion shows eosinophilic infiltration with histiocytes and granular pneumocytes (H&E stain, ×400). (B) The epidermis shows parakeratosis, exocytosis of lymphocytes with spongiosis, and vacuolization. The dermis shows extravasated red blood cells and moderate perivascular lymphocytes and eosinophils (H&E stain, ×400).
References
1. Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006. 55:1–8.
2. Aihara Y, Ito SI, Kobayashi Y, Yamakawa Y, Aihara M, Yokota S. Carbamazepine-induced hypersensitivity syndrome associated with transient hypogammaglobulinaemia and reactivation of human herpesvirus 6 infection demonstrated by real-time quantitative polymerase chain reaction. Br J Dermatol. 2003. 149:165–169.

3. Talbot RG, Nimmo J, Julian DG, Clark RA, Neilson JM, Prescott LF. Treatment of ventricular arrhythmias with mexiletine (Ko 1173). Lancet. 1973. 2:399–404.
4. Higa K, Hirata K, Dan K. Mexiletine-induced severe skin eruption, fever, eosinophilia, atypical lymphocytosis, and liver dysfunction. Pain. 1997. 73:97–99.

5. Sasaki K, Yamamoto T, Kishi M, Yokozeki H, Nishioka K. Acute exanthematous pustular drug eruption induced by mexiletine. Eur J Dermatol. 2001. 11:469–471.
6. Seino Y, Yamauchi M, Hirai C, Okumura A, Kondo K, Yamamoto M, Okazaki Y. A case of fulminant type 1 diabetes associated with mexiletine hypersensitivity syndrome. Diabet Med. 2004. 21:1156–1157.

7. Sekiguchi A, Kashiwagi T, Ishida-Yamamoto A, Takahashi H, Hashimoto Y, Kimura H, Tohyama M, Hashimoto K, Iizuka H. Drug-induced hypersensitivity syndrome due to mexiletine associated with human herpes virus 6 and cytomegalovirus reactivation. J Dermatol. 2005. 32:278–281.

8. Yagami A, Yoshikawa T, Asano Y, Koie S, Shiohara T, Matsunaga K. Drug-induced hypersensitivity syndrome due to mexiletine hydrochloride associated with reactivation of human herpesvirus 7. Dermatology. 2006. 213:341–344.

9. Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, Hjorth N, Maibach HJ, Malalten KE, Meneghini CL, Pirila V. Terminology of contact dermatitis. Acta Derm Venereol. 1970. 50:287–292.
10. Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: Dress). Semin Cutan Med Surg. 1996. 15:250–257.

11. Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988. 82:1826–1832.

12. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: A marker for stevens-johnson syndrome. Nature. 2004. 428:486.
13. Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H, Graf E, Schumacher M, Hovnanian A, Mockenhaupt M, Roujeau JC. A marker for stevens-johnson syndrome...: Ethnicity matters. Pharmacogenomics J. 2006. 6:265–268.
14. Chung WH, Hung SI, Chen YT. Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2007. 7:317–323.

15. Sullivan JR, Shear NH. The drug hypersensitivity syndrome: What is the pathogenesis? Arch Dermatol. 2001. 137:357–364.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download