Abstract
A 71-yr-old male patient with three vessel coronary artery disease underwent a coronary artery bypass graft. The patient was found to have a large pericardial defect at the apex of the heart that measured approximately 18 cm in circumference. The edge of the pericardial defect impinged on the epicardial coronary arteries. The left phrenic nerve descended via the dorsal boundary of the pericardial defect. Following coronary artery bypass grafting, the pericardial defect was repaired with a polytetrafluorethylene patch. The patient had an uncomplicated postoperative course.
The congenital absence of the pericardium is a rare condition. Patients with congenital pericardial defects may present with chest pain. The exact mechanism of this chest pain has not been well-defined, but angina-like symptoms may occur when the edge of the pericardial defect compromises blood flow by impinging on the coronary arteries (1). Establishing a proper diagnosis and management strategy can be difficult, particularly for those patients who have concomitant atherosclerotic coronary artery disease. The course of the left phrenic nerve tends to run along the antero-medial free rim of the defect. Our case exemplifies the extremely rare finding of a phrenic nerve that runs along the postero-lateral aspect of the defect.
A 71-yr-old male hypertensive patient was admitted to the Department of Orthopedic Surgery for spondylolisthesis. The preoperative echocardiogram showed an anterior wall motion abnormality. A coronary angiogram was performed, which demonstrated 95% stenosis of the middle left anterior descending coronary artery (LAD), 95% stenosis of the proximal left circumflex artery (LCX), and 90% stenosis of the right coronary artery (Fig. 1). The chest radiography was normal. An electrocardiogram showed a nonspecific ST-T abnormality.
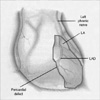
We then performed coronary artery bypass grafting. The patient was found to have a large pericardial defect measuring approximately 18 cm in circumference at the apex of the heart. The stenotic areas along the coronary arteries were located directly beneath the rim of the pericardial defect. The heart was tethered in place by adhesions along its anterior surface (Fig. 2). Once these adhesions were lysed, the heart easily herniated approximately two-thirds of its mass through the pericardial defect. The left phrenic nerve descended via the dorsal boundary of the pericardial defect. Coronary artery bypass graft was performed; left internal thoracic artery (LITA) to LAD and composite Y saphenous graft to distal LCX and posterior descending artery.
Following coronary artery bypass graft, the pericardial defect was repaired with a polytetrafluorethylene patch. The patient had an uncomplicated postoperative course, and he is currently pain-free at his 3-month follow-up.
Congenital pericardial defects are rare, with fewer than 200 cases reported in the literature. Two types of congenital pericardial defects exist: a complete form and a partial form. These defects occur more commonly on the left side of the pericardium (1).
With regard to etiology, it is widely held that this disorder represents the persistence of the embryonic pleuro-pericardial foramen (2). Its persistence may be due to inadequate blood supply following premature atrophy of the left common cardinal vein (the duct of Cuvier), which is an event that could account for both the defect and its tendency to occur on the left side (2). A recent article suggests that some defects may be due to a tear in the pleuro-pericardial membrane rather than failure of the pleuro-pericardial foramen to close (3).
The most common symptom reported by patients with congenital pericardial defects is chest pain. The mechanism of this chest pain is postulated to be due to 1) impingement of the fibrous pericardial rim on the coronary arteries, causing myocardial ischemia; 2) torsion of the great vessels; 3) lack of a cushioning effect of the pericardium; and 4) tension on the pleuropericardial adhesions (4).
The diagnosis of a congenital pericardial defect may be difficult to establish. In some cases, chest radiography can show a prominence in the hilar region, but it has been reported that magnetic resonance imaging and direct intraoperative findings are more reliable for making an exact diagnosis (5). It can be particularly difficult to establish a proper diagnosis and management strategy, including surgical intervention, in patients with concomitant atherosclerotic coronary artery disease (1). Coronary angiography may demonstrate dynamic migratory obstructions that are suggestive of a pericardial defect (6).
Complete pericardial defects require no intervention unless complications occur. For example, patients with debilitating symptoms may benefit from pericardioplasty (7). Extension of the defect by pericardiectomy or pericardioplasty may be performed when a partial defect shows evidence of herniation or is at risk of herniating. Excision of the left atrial appendage may be necessary if it herniates (8). Postoperative adhesions may also help stabilize the heart. As reported in the literature, while the course of the left phrenic nerve varies with the location and size of the foramen, it tends to run along the anteromedial free rim of the defect. It has rarely been reported that the left phrenic nerve runs behind it (9, 10). Kaneko and associates (3) report a patient with a complete left pericardial defect whose phrenic nerve, split into two portions, passes both ventral and dorsal to the defect. Our case is an example where the phrenic nerve runs behind the defect.
Figures and Tables
Fig. 1
(A) Antero-posterior view of coronary angiography showing compression of the mid-left anterior descending artery (arrow). (B) Right antero-oblique view of coronary angiography showing stenosis of the proximal left circumflex artery (arrow).

Fig. 2
Schematic drawing depicting the intra-operative findings. As the heart herniated into the left pleural cavity, it was strangulated by the margins of the remaining pericardium, resulting in a bridle stricture of both the right and left margins of the heart, with subsequent narrowing of the ventricle in this area
References
1. Nguyen DQ, Wilson RF, Bolman RM 3rd, Park SJ. Congenital pericardial defect and concomitant coronary artery disease. Ann Thorac Surg. 2001. 72:1371–1373.

2. Deutsch V, Yahini JH, Shem-Tov A, Neufeld HN. Congenital pericardial defect. Br J Radiol. 1970. 43:67–71.

3. Kaneko Y, Okabe H, Nagata N. Complete left pericardial defect with dual passage of the phrenic nerve: a challenge to the widely accepted embryogenic theory. Pediatr Cardiol. 1998. 19:414–417.

4. Lajos TZ, Bunnell IL, Colokathis BP, Schimert G. Coronary artery insufficiency secondary to congenital pericardial defect. Chest. 1970. 58:73–76.

5. Gassner I, Judmaier W, Fink C, Lener M, Waldenberger F, Scharfetter H, Hammerer I. Diagnosis of congenital pericardial defects, including a pathognomic sign for dangerous apical ventricular herniation, on magnetic resonance imaging. Br Heart J. 1995. 74:60–66.

6. Risher WH, Rees AP, Ochsner JL, McFadden PM. Thoracoscopic resection of pericardium for symptomatic congenital pericardial defect. Ann Thorac Surg. 1993. 56:1390–1391.

7. Gatzoulis MA, Munk MD, Merchant N, Van Arsdell GS, McCrindle BW, Webb GD. Isolated congenital absence of the pericardium: clinical presentation, diagnosis, and management. Ann Thorac Surg. 2000. 69:1209–1215.

8. Robin E, Ganguly SN, Fowler MS. Strangulation of the left atrial appendage through a congenital partial pericardial defect. Chest. 1975. 67:354–355.

9. Bernal JM, Lapiedra JO, Gonzalez I, Saez A, Pastor E, Miralles PJ. Angiocardiographic demonstration of a partial defect of the pericardium with herniation of the left atrium and ventricle. . J Cardiovasc Surg (Torino). 1986. 27:344–346.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download