Abstract
The aim of this study was to assess immunohistochemical expression of p53, pRb, p16, and cyclin D1, alone or in combination, as prognostic indicators and to investigate their correlation with clinocopathologic features of urothelial carcinoma. Immunohistochemical staining for p53, pRb, p16, and cyclin D1 was performed on a tissue microarray from 103 patients with urothelial carcinoma who underwent radical cystectomy. Of the patient samples analyzed, 36 (35%), 61 (59%), 47 (46%) and 30 (29%) had altered expression of p53, pRb, p16, and cyclin D1, respectively. Abnormal expression of p53 and pRb correlated with depth of invasion (P=0.040 and P=0.044, respectively). Cyclin D1 expression was associated with tumor stage and recurrence (P=0.017 and P=0.036, respectively). Altered pRb was significantly correlated with overall survival (P=0.040). According to the expression pattern of pRb and p53, p53/pRb (altered/normal) had worse survival than p53/pRb (normal/altered) (P=0.022). Alteration of all markers had worse survival than all normal (P=0.029). As determined by multivariate analysis, tumor stage, lymph node metastasis and the combined expression of p53 and pRb are independent prognostic factors. In conclusion, immunohistochemical evaluation of cell cycle regulators, especially the p53/pRb combination, might be useful in planning appropriate treatment strategies.
Bladder cancer is the second most common cancer of the genitourinary tract and accounts for about 3.2% of all cancers worldwide with about 336,000 new cases diagnosed annually (1). In Korea, the incidence of bladder cancer has been steadily increasing in recent years; currently, more than 2,200 new cases are diagnosed annually (2). Bladder cancer is also the second most common malignancy of the genitourinary tract in Korea (3). About 95% of bladder tumors are of epithelial origin, and most epithelial tumors are urothelial tumors. About 15-30% of all patients with bladder cancer are initially diagnosed with muscle-invasive or advanced disease, which has a 50% 5-yr mortality rate (4).
Stage, lymph node metastasis and grade are well-documented conventional prognostic factors for bladder cancer, but these conventional factors are inadequate to successfully predict which patients will experience recurrence and/or metastasis (5). The identification of adjunctive molecular markers may assist clinical decision-making. Cell proliferation via loss of cell cycle regulation is the one of the most important molecular and genetic changes in bladder carcinoma (6).
Herein, we evaluated candidate biomarkers associated with the G/S cell cycle checkpoint, including pRb, p53, p16 and cyclin D1, in archived paraffin-embedded urothelial carcinoma tissue.
We analyzed 103 patients who underwent cystectomy for histologically confirmed urothelial carcinoma between January 1996 and December 2005 at the Kangnam St. Mary's Hospital and St. Mary's Hospital at the Catholic University of Korea. Approval (KC10SISI0155) was obtained from the institutional review board of the Catholic University of Korea, Seoul St. Mary's Hospital.
In order to construct the tissue microarray block, 3 mm-sized core biopsies were taken from viable morphologically representative areas of the paraffin-embedded tumor tissue and were assembled on a recipient paraffin block containing 30 biopsies. This was carried out using a precision instrument (Micro Digital Co., Gunpo, Korea). After construction, 5 µm sections were cut and the histology was verified by hematoxylin-eosin staining. Each of the recipient blocks had 3 different control cores. The control cores consisted of a normal urinary bladder tissue, a normal palatine tonsil, and a breast invasive ductal carcinoma.
Five-micrometer sections of the paraffin-embedded tissue arrays were cut and mounted on sialanized glass slides. After deparaffinization, they were rehydrated in a graded series of alcohol. Heat-induced epitope retrival was conducted by immersing slides in Coplin jars filled with 10 mM/L citrate buffer (pH 6.0) and boiled in a microwave vacuum histoprocessor (RHS-1, Milestone, Pergamo, Italy) at a controlled final temperature of 121℃ for 15 min and then cooling to room temperature for 15 min. After the epitope retrieval, slides were treated with 3% H2O2 in methanol for 10 min at room temperature to abolish endogenous peroxidase activity. The tissue arrays were processed in an automatic IHC staining machine using standard protocols (Lab Vision autostainer, Lab Vision Co., Fremont, CA, USA) with a DAKO ChemMate™ EnVision™ system (DAKO, Carpinteria, CA, USA). The following antibodies were used: pRb (1:200, MAb1, Zymed Laboratories Inc., San Francisco, CA, USA), p53 (1:50, DO-1, Immunotech, Marseille, Cedex, France), p16 (1:200, E6H4, DAKO) and cyclin D1 (1:50, P2D11F11, Novocastra Laboratories Ltd, Newcastle upon Tyne, UK). The immunoreactions was developed with 3-3'-diaminobenzidine (DAB, DAKO) for 5 min, and then counterstained with Mayer hematoxylin.
Immunostaining for pRb, p53, p16 and cyclin D1 was independently examined by two pathologists. Firstly, as previously reported by Im et al. (7), expression levels of pRb, p53, p16 and cyclin D1 were determined semi-quantitatively based on the fraction of tumor cells showing positive nuclear staining (grade 0, -10%; grade 1+, 11-25%; grade 2+, 26-50%; grade 3+, 51-75%; grade 4+, 76-100%). For the purpose of further analysis, all markers were placed in one of two categories, altered or normal. Nuclear p53 and cyclin D1 were considered altered when more than 10% of tumor cell nuclei (grade 1+, 2+, 3+, 4+) showed immunoreactivity (8, 9) (Fig. 1). Tumor samples with negative (grade 0) and homogenous strong nuclear positive staining (grade 4) for pRb and p16 were considered altered as previously reported by Shariat et al. (10-12) and Chatterjee et al. (13) (Fig. 1).
All statistical analyses were performed using the SPSS program (version 11.5) for Windows. Survival duration was defined as the time from surgery to death attributed to bladder cancer. The association between IHC results and clinicopathological variables was evaluated using the Spearman correlation test and chi-squared-test. Survival curves were plotted using the Kaplan-Meier method, and statistical significance was determined by the log-rank test. Univariate analysis and multivariate survival analysis were performed using the Cox proportional hazards model. P value of <0.05 was considered significant.
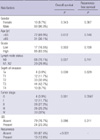
Patients included 93 males and 10 females, and their ages at the time of surgery ranged from 27 to 87 yr (mean of 67 yr and median of 68 yr). Seventeen patients (16.5%) had low-grade urothelial carcinoma and 86 patents (83.5%) had high-grade urothelial carcinoma. Of the total patient group, 4 patients (3.9%), 12 patients (11.7%), 28 patients (27.2%), 26 patients (25.2%), and 33 patients (32%) were stage 0, 1, 2, 3, and 4, respectively. Twenty-nine patients (29.9%) had lymph node metastasis at cystectomy. Through the review of whole slides, urothelial carcinoma in situ was observed in 24 cases (23.3%). Median length of follow-up was 31.5 months (range 2-133 months). Within the observation period, a total of 46 patients died from cancer-related causes. Clinical characteristics of all 103 patients are summarized in Table 1. Univariate survival analysis revealed that stage, lymph node metastasis, and depth of invasion were significantly associated with overall survival (P=0.001, P=0.037, and P=0.038, respectively). However, all factors were not statistically significantly associated with time to recurrence.
A total of 42 (40.8%), 24 (23.3%), 23 (22.3%), 9 (8.7%) and 5 (4.9%) tumors had 0, 1+, 2+, 3+, 4+ staining for p16, respectively. By classifying tumors as having normal (1+, 2+, 3+) or altered (0, 4+) expression of p16, we considered 53% of tumors as wild-type expression and 47% of tumors as altered expression. A total of 51 (49.5%), 20 (19.4%), 9 (8.7%), 13 (12.6%) and 10 (9.7%) tumors had 0, 1+, 2+, 3+, and 4+ staining for pRb, respectively. By classifying tumors as having altered (0, 4+) or normal (1+, 2+, 3+) expression of pRb, we considered 41% of tumors as normal expression and 59% of tumors as altered expression. A total of 73 (70.9%), 10 (9.7%), 12 (11.7%), 8 (7.8%), and 0 (0%) tumors had 0, 1+, 2+, 3+, and 4+ staining for cyclin D1, respectively. By classifying tumors as having normal (0) or altered (1+, 2+, 3+, 4+) expression of cyclin D1, we considered 71% of tumors as negative expression and 29% of tumors as altered expression. A total of 51 (49.5%), 5 (4.9%), 16 (15.5%), 12 (11.7%), and 19 (18.4%) tumors had 0, 1+, 2+, 3+, and 4+ staining for p53, respectively. By classifying tumors as having normal (0) or altered (1+, 2+, 3+, 4+) expression of p53, we considered 65% of tumors as negative expression and 35% of tumors as altered expression.
There was a significant relationship between expression of pRb and cyclin D1 (P=0.029 and r=0.215) and between expression of p16 and p53 (P=0.036 and r=0.207). There was a significant inverse relationship between expression of cyclin D1 and p16 (P=0.001 and r=-0.320) (Table 2).
We tested for correlations between the expression of pRb, p53, p16 and cyclin D1 and clinicopathological parameters, such as depth of invasion, lymph node metastasis, tumor stage, tumor grade, presence of urothelial carcinoma in situ (UCIS) component and recurrence (Table 3). Altered expression of cyclin D1 was associated with recurrence and higher tumor stage (P=0.036 and P=0.017, respectively). Altered expression of pRb and p53 correlated significantly with depth of tumor invasion (P=0.044 and P=0.040, respectively). There was no specific correlation between the expression of p16 and these clinicopathologic parameters. Kaplan-Meier univariate analysis demonstrated worse survival with altered expression of pRb (P=0.040) (Fig. 2). Altered expression of p53, p16 and cyclin D1 did not have a significant impact on patient survival time (P=0.123, P=0.804, P=0.741, respectively), although mean survival time with altered expression of cyclin D1 was reduced (data not shown). Evaluation of the combined status of markers, which included six possible combinations (pRb/p53, pRb/p16, pRb/cyclin D1, p53/p16, p53/cyclin D1, and p16/cyclin D1) revealed that only pRb/p53 had a statistically significant impact on overall patient survival (P=0.011). We analyzed overall survival time with respect to the expression pattern of pRb and p53 (Table 4). This analysis indicates that patients whose tumors had pRb/p53 (altered/normal) expression had worse survival than those whose tumors had pRb/p53 (normal/altered) expression (P=0.022) (Fig. 3). We also analyzed overall survival time with respect to the number of altered markers (i.e. none, one, two, three, or four altered). We found that patients whose tumors had altered expression of all four markers had worse survival than those whose tumors had altered expression of none (P=0.029) (Fig. 4). We also found that patients whose tumors had altered expression of two markers appeared to have decreased survival compared to those whose tumors had altered expression of only one marker; however, this observation was of borderline statistical significance (P=0.052). We performed Cox multivariate analysis with various clinicopathologic variables (Table 5). Tumor stage, lymph node metastasis and the combined expression of pRb and p53 were identified as independent prognostic factors.
The prediction of which superficial bladder tumors will recur or progress and which advanced tumors will metastasize and prove fatal to the patient remains a substantial challenge to be addressed in bladder cancer treatment. Despite great advances in our understanding of urinary bladder carcinogenesis, attempts to identify molecular prognostic or predictive factors other than the conventional clinical indicators, such as tumor stage and grade, have been largely unsuccessful. Molecular changes in bladder tumors involve three main mechanisms: chromosomal alteration (the initial event in carcinogenesis), tumor proliferation due to loss of cell cycle regulation, and metastasis aided by processes such as angiogenesis and the loss of cell adhesion (6).
Aberrations in G1/S regulatory proteins are common in various tumors, and aberrant expression of cyclin D1 and cyclin E, down-regulation of p16 and p27, and mutation of the Rb and p53 genes have been frequently observed in several types of cancer. Therefore, it has been suggested that G1/S defects might be obligatory for tumor development (14). Perhaps because of multiple redundant pathways that exist to stimulate downstream effectors, there are inconsistent results in the literature concerning the use of a single marker of cell cycle regulation as a prognostic factor in urothelial carcinoma (15). Therefore, several studies have suggested the possibility of cooperative effect involving multiple cell cycle regulators (10, 11, 13, 16).
In Korea, prognostic significance of p53, p21 and pRb in urothelial carcinoma was reported by Cho et al. (17). They analyzed the relationship between recurrence and progression and the results of immunostaining in a T1G3 bladder cancer. Any single marker did not correlate with tumor recurrence or progression. A combination of altered immunostaining for p53/p21/pRb (+/-/-) correlated with progression but not with recurrence. But, it included only 30 pTl high grade urothelial carcinomas without survival analysis. Despite marked differences in the prognosis of pT1 and pT2-4 cancers, these tumors are highly similar on the genetic level (18, 19). It could be expected, that similar genetic alterations might be prognostically relevant in all stages. In this present study, we included early and advanced carcinoma and found that the combined expression of pRb and p53 was an independent prognostic factor, and patients whose tumors had altered expression of all four markers had significantly worse survival compared to those whose tumors had altered expression of none. Patients whose tumors had altered expression of two markers appeared to have diminished survival compared to those whose tumors had altered expression of one marker, but this observation was of borderline statistical significance. The results of our study support the hypothesis that cell cycle regulators synergistically affect the progression of urothelial carcinoma.
Out of all four markers, only pRb had a significant impact on patient survival time in our study. Expression of pRb was altered in 59% of cases, which is slightly higher than the 50% of cases reported by Cote et al. (8) and 55% of cases reported by Chatterjee et al. (13). Previous reports considered negative or strong positive (>50%) staining as altered pRb expression because it has been shown that overexpression of pRb in bladder cancer is also indicative of dysfunctional pRb status (8). Absence of pRb reactivity is indicative of loss of gene expression, generally through mutation (20), and pRb overexpression reflects an alteration in the Rb pathway resulting in loss of several cyclin dependent kinase inhibitors. Therefore both loss and overexpression of pRb and p16 were considered altered (8, 21).
We modified these criteria. Negative or strong positive (>75%) staining was considered to be altered expression of pRb. Because we used microarray tissue blocks, we evaluated limited areas of tumor compared to previous reports. Although stricter criteria was applied, expression of pRb was not significantly different from that found in other studies.
Altered expression of all four markers, regardless of pathologic stage, correlated with decreased survival compared to normal expression. This finding, therefore, suggests that multiple genetic defects affect the progression and metastasis of urothelial carcinoma.
There are two distinct precursor lesions to invasive urothelial carcinoma: non-invasive papillary tumors (NIPT) and flat non invasive urothelial carcinoma (CIS) (22, 23). Whether CIS was present or not (CIS present vs. CIS absent) and whether NIPT was present or not (NIPT present vs. NIPT absent), there was no significant difference in the status of cell cycle markers (data not shown). However, it was a limited result because precursor lesions were not detected in some cases. Generally, in about one half of individuals with invasive bladder cancer, the tumor has already invaded the bladder wall. At the time of presentation, no precursor lesions can be identified because the high-grade invasive component extensively replaces normal structure and appears as a large ulcerated mass.
There was a significant correlation between the expression of certain markers (pRb and cyclin D1, p16 and p53, cyclin D1 and p16). This suggests a close relationship between these pairs of cell cycle regulators. However, these combinations of markers demonstrated no significant association with survival or clinicopathologic factors. It is well known that high expression of p16 can lead to loss of the pRb protein, and it has been reported that these two factors are inversely correlated in urothelial carcinoma (10). Benedict et al. (21) reported there was relationship between LOH at 9p21 ( the locus of the MTS-1/INK 4a gene encoding for p16) and/or homozygous deletions/mutations within the MTS-1 gene and p16 or pRb status as determined by immunohistochemistry. They suspected that the immunohistochemical results of p16 could be the reflection of the genetic alteration status. Our results demonstrated an inverse relationship between expression of p16 and pRb, but this correlation was not statistically significant.
In conclusion, analysis of cell cycle regulators provides prognostic information in addition to that which can be derived from well-known prognostic factors, such as grade and stage. Therefore, immunohistochemical evaluation of cell cycle regulators, especially the p53/pRb combination, should be used as a predictive tool and might provide clinicians with useful information to determine treatment strategy.
Figures and Tables
Fig. 1
Immunohistochemical expression. (A, B) pRb strong homogenous positive and negative (pRb, ×400), (C, D) p16 homogenous positive and negative (p16, ×400), (E) p53 positive (p53, ×400), (F) cyclin D1 positive (Cyclin D1, ×400).
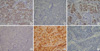
Fig. 3
Overall survival curves according to combined p53 and pRb status (P value from log rank test).

Fig. 4
Overall survival curves according to the number of altered markers (P value from log rank test).

Table 1
Univariate analysis for overall survival and recurrence-free survival in 103 urothelial carcinoma
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.

2. Jung KW, Won YJ, Park S, Kong H, Sung J, Shin H, Park E, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.

3. Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual Report of the Korea Central Cancer Registry: Based on Registered Data from 139 Hospitals. Cancer Res Treat. 2004. 36:103–114.

4. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. The world health organization classification of tumors of the urinary system and male genital system. 2004. Lyon: IARC press;92–109.
5. Margulis V, Lotan Y, Montorsi F, Shariat SF. Predicting survival after radical cystectomy for bladder cancer. BJU Int. 2008. 102:15–22.

7. Im S, Yoo C, Jung JH, Choi HJ, Yoo J, Kang SJ, Lee KY. Alteration of G1/S cell cycle regulatory proteins in carcinogenesis of cutaneous squamous cell carcinomas. Korean J Pathol. 2009. 43:542–549.

8. Cote RJ, Dunn MD, Chatterjee SJ, Stein JP, Shi SR, Tran QC, Hu SX, Xu HJ, Groshen S, Taylor CR, Skinner DG, Benedict WF. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998. 58:1090–1094.
9. Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Association of cyclin D1 and E1 expression with disease progression and biomarkers in patients with nonmuscle-invasive urothelial cell carcinoma of the bladder. Urol Oncol. 2007. 25:468–475.

10. Shariat SF, Tokunaga H, Zhou J, Kim J, Ayala GE, Benedict WF, Lerner SP. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004. 22:1014–1024.

11. Shariat SF, Zlotta AR, Ashfaq R, Sagalowsky AI, Lotan Y. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod Pathol. 2007. 20:445–459.

12. Shariat SF, Chade DC, Karakiewicz PI, Ashfaq R, Isbarn H, Fradet Y, Bastian PJ, Nielsen ME, Capitanio U, Jeldres C, Montorsi F, Lerner SP, Sagalowsky AI, Cote RJ, Lotan Y. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J Urol. 2010. 183:68–75.

13. Chatterjee SJ, Datar R, Youssefzadeh D, George B, Goebell PJ, Stein JP, Young L, Shi SR, Gee C, Groshen S, Skinner DG, Cote RJ. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004. 22:1007–1013.

14. Loden M, Stighall M, Nielsen NH, Roos G, Emdin SO, Ostlund H, Landberg G. The cyclin D1 high and cyclin E high subgroups of breast cancer: separate pathways in tumorogenesis based on pattern of genetic aberrations and inactivation of the pRb node. Oncogene. 2002. 21:4680–4690.

15. Knowles MA. What we could do now: molecular pathology of bladder cancer. Mol Pathol. 2001. 54:215–221.

16. Hitchings AW, Kumar M, Jordan S, Nargund V, Martin J, Berney DM. Prediction of progression in pTa and pT1 bladder carcinomas with p53, p16 and pRb. Br J Cancer. 2004. 91:552–557.

17. Cho SY, Kim YS, Hong SJ. Prognostic significance of p53, pRb, and p21 (waf1) in T1G3 bladder cancer. Korean J Urol. 2002. 43:678–682.
18. Richter J, Beffa L, Wagner U, Schraml P, Gasser TC, Moch H, Mihatsch MJ, Sauter G. Patterns of chromosomal imbalances in advanced urinary bladder cancer detected by comparative genomic hybridization. Am J Pathol. 1998. 153:1615–1621.

19. Simon R, Burger H, Semjonow A, Hertle L, Terpe HJ, Bocker W. Patterns of chromosomal imbalances in muscle invasive bladder cancer. Int J Oncol. 2000. 17:1025–1029.

20. Xu HJ, Cairns P, Hu SX, Knowles MA, Benedict WF. Loss of RB protein expression in primary bladder cancer correlates with loss of heterozygosity at the RB locus and tumor progression. Int J Cancer. 1993. 53:781–784.

21. Benedict WF, Lerner SP, Zhou J, Shen X, Tokunaga H, Czerniak B. Level of retinoblastoma protein expression correlates with p16 (MTS-1/INK4A/CDKN2) status in bladder cancer. Oncogene. 1999. 18:1197–1203.

22. Ebstein JI. Kumar V, Abbas AK, Fausto N, Aster JC, editors. The lower urinary tract and male genital system. Pathologic Basis of Disease. 2010. 8th ed. Philadelphia: Elsevier;976–980.
23. Amin MB, Gómez JA, Young RH. Urothelial transitional cell carcinoma with endophytic growth patterns: a discussion of patterns of invasion and problems associated with assessment of invasion in 18 cases. Am J Surg Pathol. 1997. 21:1057–1068.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download