Abstract
This study was done to evaluate the stemness of human mesenchymal stem cells (hMSCs) derived from placenta according to the development stage and to compare the results to those from adult bone marrow (BM). Based on the source of hMSCs, three groups were defined: group I included term placentas, group II included first-trimester placentas, and group III included adult BM samples. The stemness was evaluated by the proliferation capacity, immunophenotypic expression, mesoderm differentiation, expression of pluripotency markers including telomerase activity. The cumulative population doubling, indicating the proliferation capacity, was significantly higher in group II (P<0.001, 31.7±5.8 vs. 15.7±6.2 with group I, 9.2±4.9 with group III). The pattern of immunophenotypic expression and mesoderm differentiation into adipocytes and osteocytes were similar in all three groups. The expression of pluripotency markers including ALP, SSEA-4, TRA-1-60, TRA-1-81, Oct-4, and telomerase were strongly positive in group II, but very faint positive in the other groups. In conclusions, hMSCs from placentas have different characteristics according to their developmental stage and express mesenchymal stemness potentials similar to those from adult human BMs.
Human mesenchymal stem cells (hMSCs) are multipotent adult stem cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes, adipocytes, neuronal cells, and beta-pancreatic islets cells. hMSCs have been identified in many human adult organs (e.g, bone marrow, peripheral blood, cornea/retina, brain, skeletal muscle, dental pulp, liver, and adipose tissues) as well as in the fetus, including the liver, bone marrow, kidney, umbilical vein endothelial/subendothelial layer, and in the blood of preterm fetuses (1-4). Human bone marrow (hBM) has been the main source of hMSCs, sometimes referred to as bone marrow stromal cells because of their characteristics and capacity to transform into mesodermal tissues, such as adipocytes, osteoblasts, and chondrocytes. However, the use of hBM as the source for hMSCs is limited by the invasive collection procedure required as well as a decrease in cell number, proliferation, and differentiation capacity in vitro with the age of the culture (5). This has led many researchers to investigate alternate sources of hMSCs that can be used in the clinical setting.
Human umbilical cord blood (hUCB), which is an increasingly accepted source for hematopoietic stem cell transplantation in children and adults (6), has been considered as an alternative source of hMSCs. However, whether hUCBs is an acceptable source of hMSCs continues to be investigated. Some studies have reported that hMSCs were not present in the hUCB from term deliveries and were identified only in fetal blood (7). Recently, the presence of hMSCs in the human placenta (hPC) has been confirmed and reported to be a valuable and practical source for tissue engineering (8-11). Studies that compared the characteristics of the hMSCs from hPC with those from hBM have reported different features in the hMSCs collected from the fetal side compared to those collected from the maternal side of the hPC (10, 12). Moreover, Guillot et al. (13) reported that human first-trimester fetal hMSCs express pluripotency markers and grow faster than adult hMSCs. In addition, a study showing differences between the hMSCs of fetal tissues and adult tissues was recently reported (13, 14). Furthermore, Mikkola et al. (15) showed that the preterm hPC from the first-trimester, not the term hPC, would be preferred for the study of hematopoietic stem cell development. These findings suggest the presence of some differences between the hMSCs from the preterm hPC during the first-trimester compared to those from the term hPC. However, there is no prior study comparing the features of mesenchymal and pluripotent stemness of hMSCs derived from first trimester preterm hPCs in embryonic to early fetal developmental stage, with term hPCs during the late fetal developmental stage and human adult bone marrow (hBM). Therefore, this study was designed to evaluate the proliferation capacity, phenotypic expression, mesoderm differentiation, expression of pluripotency stem cell markers including telomerase activity, and karyotype stability of hMSCs derived from term hPCs and compare the results with preterm hPCs from the first trimester and from adult hBM.
We obtained five first-trimester hPCs at 8-12 weeks gestational age from women that had undergone therapeutic abortion due to known maternal disease that is aggravated by pregnancy and five term hPCs at 38-40 weeks of gestational age from healthy pregnant women, after the acquisition of written informed consent. Loose chorion, amniotic sac, and decidua were removed from the placenta. Human chorionic plate (hCP) membranes were surgically separated from the placenta by the specialist of Obstetrics, incubated in 0.25% trypsin-EDTA (ethylene amine tetra acetic acid; GIBCO-Invitrogen, Carlsbad, CA, USA) at 37℃ for 30 min, and then washed with PBS. Thereafter, the cells were isolated from the hCP by digestion with 0.05% collagenase type 1 (Sigma-Aldrich, St. Louis, MO, USA) at 37℃ for 30 min and washed three times with PBS. Adult hBM was obtained from five healthy males that voluntarily donated their hBM for allogeneic hematopoietic stem cell transplantation for which they gave written informed consents. Mononuclear cells (MNCs) were isolated from heparinized hBM aspirates (diluted with equal volume of PBS) by standard density (1.077 g/mL) centrifugation using Ficoll (Sigma-Aldrich, St.Louis, MO, USA). The MNCs at the interface were recovered, washed, and resuspended in the medium composed of Dulbecco's Modified Eagle's Medium (DMEM) with low glucose, 10% fetal bovine serum (FBS), and 1% antibiotic-antimycotic stock solution (all from GIBCO-Invitrogen). This study was conducted prospectively, with the approval of the Institutional Review Board for human research in Korea University Medical Center (IRB approval number: AN09085-001) and all patients provided written informed consent.
All of the collected tissues were microbiologically evaluated before culture, because hMSCS infected by pathogens should not be clinically applied. The samples were tested for bacteria, Chlamydia spp., Cytomegalovirus, Herpes simplex virus types 1 and 2, Mycoplasma spp., and Ureaplasma urealyticum. In addition, Mycoplasma spp. detection was assessed by the polymerase chain reaction (PCR) with branded products. The PCR Mycoplasma detection set (TaKaRa®, Shiga, Japan ) is a primer set designed to detect the presence of Mycoplasma (M. fermentans, M. hyorhinis, M. arginini, M. orale, M. salivarium, M. hominis, M. pulmonis, M. arthritidis, M. neurolyticum, M. hyopneumoniae, M. capricolum) and one species of Ureaplasma (U. urealyticum).
Evaluation of chromosome stability was performed at every fifth passage. The hMSCs were incubated with 0.1 µg/mL colcemid for three to four hours, trypsinized, and then incubated in 0.075 M KCL for 20 min at 37℃. After fixation with 3:1 methanol/acetic acid, the karyotypes of the hMSCs were determined at the 300-band level of resolution. The tissues showing abnormal karyotypes would have been excluded from this study, but no abnormal karyotypes were detected.
Isolated normal cells were placed into 175-cm2 flasks and long term cultures established using DMEM (GIBCO-Invitrogen) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories Inc, Logan, UT, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37℃, with 5% CO2 and 95% humidity. The medium was changed after 48 hr and every 3-4 days thereafter. When the cultures (the primary passage) reached approximately 90% confluence, hMSCs were recovered by digestion with 0.05% trypsin/0.53 mM EDTA solution (GIBCO-Invitrogen) and replated in passage culture at a density of about 5,000 cells per cm2. Once confluent, the harvested (passage 1) cells were similarly prepared and seeded to obtain passage two (P2) cells and so on. Based on the source of the hMSCs, three groups were established: group I included samples from term placentas, group II from preterm placentas, and group III from adult bone marrow.
To examine the proliferation capacity of the hMSCs in vitro, the cumulative population doubling (PD) was measured over 50 days. The hMSCs were plated in triplicate at a concentration of 104 cells per cm2 in 10-cm2 dishes and successively subcultured at the same density. The cells were counted using a hemocytometer and trypsin blue was used to exclude dead cells; this process was serially repeated three times over 50 days. The cumulative cell doubling of the cell populations was plotted against time in the culture to determine the growth kinetics of hMSC expansion. The number of population doubling was determined by counting the number of adherent cells at the start and end of each passage. The population doubling was calculated at every passage according to the equation: log2 (the number of harvested cells/the number of seeded cells). The finite population doubling was determined by the cumulative addition of the total numbers generated from each passage until the cells stopped dividing.
To study the morphological characteristics of the cells, the cell culture flasks were examined at all medium re-feeding intervals (every 4 days), to detect any abnormalities in cell morphology and/or medium. When any variation was observed, the changes were recorded in the culture files.
The culture expanded cells were labeled with the following anti-human antibodies, used for the characterization of hMSCs, at the fifth passage: CD29-PE (phycoerythrin), CD31-FITC (fluorescein isothiocyanate), CD34-FITC, CD44-FITC, CD45-FITC, CD90-FITC, CD105-FITC, HLA-ABC-PE and HLA-DR-FITC (all Becton Dickinson [BD], San Jose, CA, USA). After exposure to labeled antibodies, the cells were washed and resuspended in ice cold PBS . More than 50,000 labeled cells were acquired and analyzed using FACS-Vantage-SE flow cytometry running Cell-Quest software (BD). Negative control samples were stained with isotype-matched irrelevant monoclonal antibodies. The data were analyzed with Cellquest software.
This study was performed on the hMSCs at the fifth passage. Osteogenic differentiation was induced using hMSCs medium with osteogenic supplements. For the in vitro osteogenic assays, the hMSCs were plated at densities of 3.1×103 cells/cm2 in 0.2 mL/cm2 of medium on tissue culture dishes. The following day (day 0), the culture dish was replaced with osteogenic medium (100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 M L-ascorbic acid 2-phosphate), which was changed twice a week until day 21. The hMSC medium served as the control. After 21 days, Von Kossa and alkaline phosphate staining were used to demonstrate the presence of calcium phosphate.
To induce adipogenic differentiation, the cells were plated at 1 to 2×104 cells/cm2 and cultured in DMEM (low glucose) plus 10% FBS and fed. Every 2-3 days, the medium was replaced until the cultures reached confluence (5-10 days) in the 35-mm diameter plastic dishes (BD). At 100% confluence, the cultures were treated with an adipogenic-induction medium composed of DMEM (low glucose) plus 10% FBS containing 0.5 mM isobutyl-methylxanthine (IBMX), 1 µM dexamethasone, 10 µg/mL insulin and 0.2 mM indomethacin for 3 days, followed by the maintenance medium composed of DMEM (high glucose) containing 10 µg/mL insulin for 3 days. Adipogenic differentiation was assessed using Oil Red-O stain as an indicator of intracellular lipid accumulation.
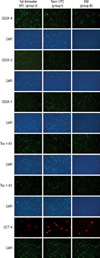
Immunofluorescence histochemistry examinations with pluripotency markers expressed in human embryonic stem cells (hESCs), including SSEA-1, SSEA-3, SSEA-4 (Hybridoma Bank, University of Iowa, IA, USA), tumor rejection antigen (TRA)-1-60, TRA-1-81 (Chemicon, Temecula, CA, USA) and OCT-4 were performed on the hMSCs at the fifth passage. The hMSCs were washed twice with PBS and fixed with 100% ethanol for 2 min. Cells were then washed with 1% BSA(Bovine serum albumin, Sigma)/PBS and incubated for 1 hr at room temperature with 100 mL of undiluted antibody against SSEA-1, SSEA-3, SSEA-4, TRA-1-60, TRA-1-81 and OCT-4. The cells were washed twice with 1% BSA/PBS and incubated in the dark with a 1:500 dilution of goat anti-mouse PE-conjugate for another hour. Simultaneously, the nuclei of the cells were stained with a 1:1,000 dilution DAPI (4',6-diamidino-2-phenylindole) solution. The cells were washed twice and sufficient 1% BSA/PBS was added to cover the cells. UV filtered images were then taken under the IX70 Olympus microscope at 100×magnification.
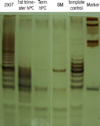
To compare the undifferentiated state of the hMSCs, we performed RT-PCR for Oct-4 and telomerase, which is highly expressed in undifferentiated stem cells. Oct-4 is a marker for the undifferentiated status of stem cells and is expressed in pluripotent cell populations, such as early embryonic cells. Its expression can influence the status of pluripotency. For reverse transcriptase (RT), first strand complementary DNA was synthesized from 2 µg of total RNA and the polymerase chain reaction (PCR) was performed using 0.2 µg of 1:10 diluted cDNA on an Bioer XP cycler (Bioer Technology Co. LTD. Hangzhou, Zhejiang, China) in a 50 µL reaction mixture that contained 10 pM of forward and reverse primers (sequences are given in Table 1), 10×PCR buffer, dNTP mixture 10 mM, and 2.5 U Taq polymerase (GENENMED, Seoul, Korea). Reaction PCR products were separated by gel electrophoresis using a 1.5% agarose gel. Bands were visualized by UV illumination of ethidium-bromide-stained gels. Band intensity was normalized to corresponding β-actin values. Telomerase activity is high in hESCs, but rarely detectable in hMSCs from adult tissues. We have conducted telomeric repeat amplication protocol (TRAP) to evaluate telomerase activity of MSCs derived from human placenta and adult BM.
The comparative growth kinetics of the hMSCs involved in this study over 50 days at a cell density of 104 cells per cm2 showed that the hMSCs of group II had a statistically higher growth potential of cumulative PDs, achieving 31.7±5.8, whereas those of the groups I and II were 15.7±6.2 and 9.2±4.9, respectively (P<0.001); with cumulative PDs, progressively increasing in group II, but slowing in groups I and III. The hMSCs of group II from the first-trimester preterm hPCs underwent up to 20 passages and displayed no visible changes with regard to their morphology by light microscopy, forward and side scatter properties on flow cytometry, or growth patterns. The numbers of possible passages for subcultures maintaining the initial morphology of groups I and II were 14.2±3.3 and 11.8±4.1, respectively, which were both fewer than the 20 passages (Figs. 1, 2).
All mesenchymal immunophenotypic expression patterns of the cells involved in this study were the same. Flow cytometry examination revealed that all of the cells were negative for CD34 (gp105-120) and CD45 (leukocyte common antigen), indicating that these cells were not of hematopoietic origin. The cells were also negative for the matrix receptor CD31 (PECAM-1) and HLA-DR. The cells were positive for integrin CD29 (β1-integrin), matrix receptor CD44 (hyaluronate receptor), CD90 (Thy-1), CD105 (endoglin), and HLA-A, B, C (Fig. 3).
Cells derived from the first-trimester and term placentas were cultured in osteogenic media and started to aggregate in 7 days, and then made multiple colony-like nodules in 14 days (Fig. 4). Von Kossa and alkaline phosphatase staining showed osteogenic differentiation, similar to the hBM cells. Adipogenic differentiation was evaluated by adipogenic induction and maintenance media cultures. Morphologic changes of the cells from the first-trimester placenta and term placentas, as well as the cells from the hBM, included the formation of neutral lipid vacuoles, which were noted at 3 weeks and visualized by the staining with Oil-Red-O (Fig. 5).
ALP staining was strongly positive in the group II hMSCs from first-trimester hPCs. However, it was weaker and more blurred for the hMSCs in group I and III. The expression of Oct-4 and telomerase activity were clearly identified in group I , but not in group II hMSCs from the hPCs and group III hMSCs (Figs. 6, 7). All of the immunocytochemical stains for SSEA-4, TRA-1-60, and TRA-1-81 on hESCs were strongly positive in group II hMSCs from preterm hPC but faint in the hMSCs from group I and II (Fig. 8).
The purpose of this study was to evaluate and compare the mesenchymal and pluripotential stemness features of hMSCs derived from first trimester hPCs during the embryonic to early stage of development, term hPCs during the late fetal stage of development and adult human bone marrow (hBM). The results showed better proliferation potential with the expression of pluripotency markers in the hMSCs of first-trimester placentas during the embryonic to early fetal stage of development compared to the late fetal and adult stages. In addition, late fetal and adult hMSCs did not strongly express pluripotency markers such as Oct-4, SSEA-3, SSEA-4, Tra-1-60, and Tra-1-81, which suggests remarkable growth capacity. These findings are consistent with those reported by Guillot et al. on first-trimester fetal blood, liver, and bone marrow hMSCs. However, Guillot et al. (13) did not study the growth capacity or expression degree of pluripotency markers in first-trimester hPCs. Therefore, this study is the first report showing that hMSCs from first-trimester preterm hPCs express pluripotency markers. In addition, the population doubling at this stage was similar to the results of the first-trimester fetal liver, blood, and bone marrow reported by Guillot et al. (13). In this study, telomerase expression of the hMSCs from the preterm placentas of the embryo and early fetal stage was examined by the RT-PCR method. Telomerase expression in hMSCs is not expected due to the low telomerase activity at this stage of development (16). Nonetheless, telomerase expression was found in the hMSCs of the embryo and early fetal developmental stage in the preterm hPCs, but not during the late fetal and adult stages of development. This result suggests a higher telomerase activity in the hMSCs from the preterm hPCs than from the term hPCs and adult hBM. High telomerase activity in the hMSCs from the first-trimester preterm hPCs might be related to the predominant proliferation potential and possibly with pluripotency marker expression.
There have been studies showing the same phenotypic expression between hMSCs originating from fetal tissue and those from adult tissue (4, 9, 10, 17). This study also showed the same pattern of immunophenotypic expression in the hPCs, regardless of gestational age, and also in the adult hBM. The pattern and potential of mesoderm differentiation of the hMSCs to adipocytes and osteocytes from the first-trimester preterm hPCs showed no significant differences compared to the hMSCs derived from term hPCs during the late fetal stage of development and adult human bone marrow (hBM). This finding is somewhat different from those of other studies that found a higher potential for differentiation among fetal hMSCs compared to adult hMSCs (15, 18). Lee et al. (18) reported that rhesus monkey MSCs derived from early third-trimester BM showed greater proliferation and differentiation potential than those from adult BM. Guillot et al. (13) also suggested that the osteogenic efficiency of the fetal hMSCs was greater than that of adult hMSCs. The adipogenic and osteogenic differentiation was evaluated in this study, but not chondrogenic differentiation; this is because it was well known that typical mesodermal differentiation can be induced to form adipocytes and osteocytes (5, 9, 19, 20). Further studies to clarify the differentiation potentials of hMSCs originating from fetal tissues and adult tissues are needed.
To apply the products from hMSCs to human treatment, chromosomal safety must be confirmed. It is very difficult to find studies that have examined the chromosomes of the hMSCs. Human stem cells that can be used for long term passage, especially hESCs, have significant problems with increased rates of chromosome mutation. In this study, all of the chromosome examinations were normal. However, this finding does not completely eliminate the possibility of chromosome mutation in the hMSCs culture. The presence of chromosome mutation in immortalized hMSCs, which were developed to sustain the characteristics of hMSCs for long periods of time, have been reported (21, 22). Therefore, careful observation of chromosomal anomalies of the hMSCs from the preterm placenta of embryonic and early fetal stages of development, which can proliferate longer than in the late fetal and adult stages, is needed.
There was a lower infection risk, especially for Mycoplasma spp., in the human bone marrow samples than in the human placentas. This finding might be due to the difference in the procedures used for tissue acquisition. Human bone marrow is acquired using aseptic techniques in the operating room because the harvested bone marrow blood must be infused into patients. However, the handling of human placentas is somewhat crude because they are usually discarded after delivery. Therefore, human placentas must be more aseptically acquired to avoid microbiological contamination if hMSC cultures are to be derived from placentas. So, in fact human placentas acquired from caesarean section delievery might be more useful than vaginal birth.
In summary, hMSCs from first trimester preterm hPCs in the embryo and early fetal stage show higher proliferation potential with the expression of pluripotency markers and telomerase activity than term hPCs during the late fetal stage and in adult hBM. These findings might be due to the fact that early gestational placentas are closer to the embryo stage than the late full term placentas. The hMSCs from term placentas might be useful candidates for a source of hMSCs for the production of mesodermal cells without risk to donors. The results of this study demonstrate that hMSCs from the placenta have different characteristics according to developmental stage.
Figures and Tables
Fig. 1
Cells from the human placentas and human bone marrow. (A) Cells from the human term placenta. (B) Cells from the first-trimester placenta. (C) Mesenchymal stem cells(MSC) from the human bone marrow. The placenta derived cells show a fibroblastoid, spindle-shaped morphology at passage 3 on day 5. The growth pattern of the cells from the term and first-trimester placenta was same as the bone marrow derived mesenchymal stem cells. Phase contrast: magnification ×100 for all figures.

Fig. 2
Proliferation Capacity. (A) Cumulative population doublings of Mesenchymal stem cells from first-trimester human placenta (hPC), term human placenta (hPC) and Bone
marrow. (B) The possible passages for subcultures with the maintenance of initial morpholology and growth pattern of three population of mesenchymal stem cells.

Fig. 3
Immunophenotype of MSCs. BM-derived MSCs (BM-MSC), first-trimester placenta derived cells and term placenta derived cells were labeled with antibodies against the indicated antigens, and analyzed by flow cytometry. Representative histograms are demonstrated. All types of cells showed the same immunophenotypic characteristics: positive expression of CD29, CD44, CD90, CD105 and HLA-ABC as surface epitopes, and negative expression of CD31, CD34, CD45 and HLA-DR.

Fig. 4
Osteogenic differentiation from placenta derived cells. For osteogenic differentiation, first-trimester placenta derived cells were cultured for up to 3 weeks in osteogenic medium (OM). (A) First-trimester placenta derived cells aggregate in 7 days and formed nodules at 14-21 days. (B) First-trimester placenta derived cells exhibit alkaline phosphatase activity at 2 weeks. (C) Von Kossa staining-positive aggregates or nodules are found after 3 weeks, which shows significant calcium deposition in osteogenic media.

Fig. 5
Adipogenic differentiation from placenta derived cells. Adipogenic differentiation was evaluated by culturing placenta-derived cells in adipogenic induction and maintenance medium for 3 weeks and evidenced by the formation of lipid vacuoles using Oil-Red-O staining and hematoxylin counterstaining. The first-trimester placentaderived cells form lipid vacuoles at 21 day (A-F). The term placenta-derived cells formed lipid vacuoles at 21 day as well (G-L). A, B, G, H: unstained. Original magnification: A, C, G, I, ×40; B, D, H, J ×100; E, K ×200; F, L, ×400.
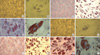
Fig. 6
The expression of Oct4 in first trimester, term placenta and BM derived Mesenchymal stem cells (MSCs). The expression of Oct-4 is strong in first-trimester hPC MSCs and weak in term placenta hPC and adult BM derived MSCs as shown by RT-PCR.
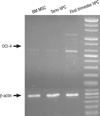
Fig. 7
The detection of telomerase activity by the TRAP assay shows the specific signal intensity of telomerase-specific 6-base repeat ladder. First lane shows the expression of telomerase activity in positive control 293T cell; 1st -trimester hPC lane shows the expression of telomerase activity; term hPC and BM MSC lane show the negative telomerase activity.
Notes
References
2. Vaanaenaen HK. Mesenchymal stem cells. Ann Med. 2005. 37:469–479.
3. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.

4. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001. 98:2396–2402.

5. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006. 7:14.
6. Barker JN, Davies SM, DeFor T, Ramsay Norma KC, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001. 97:2957–2961.

7. Yu M, Xiao Z, Shen L, Li L. Mid-trimester fetal blood-derived adherent cells share characteristics similar to mesenchymal stem cells but full-term umbilical cord blood does not. Br J Haematol. 2004. 124:666–675.

8. Haigh T, Chen C, Jones CJ, Aplin JD. Studies of mesenchymal cells from 1st trimester human placenta: expression of cytokeratin outside the trophoblast lineage. Placenta. 1999. 20:615–625.

9. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004. 22:649–658.

10. Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006. 30:681–687.

11. Zhang X, Mitsuru A, Igura K, Takahashi K, Ichinose S, Yamaguchi S, Takahashi TA. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006. 340:944–952.

12. In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004. 22:1338–1345.
13. Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007. 25:646–654.

14. Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH, Song HR, Lee JK, Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood and bone marrow. J Korean Med Sci. 2009. 24:547–554.

15. Mikkola HK, Gekas C, Orkin SH, Dieterlen-Lievre F. Placenta as a site for hematopoietic stem cell development. Exp Hematol. 2005. 33:1048–1054.

16. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006. 99:1285–1297.

17. Kim SJ, Song CH, Sung HJ, Yoo YD, Geum DH, Park SH, Yoo JH, Oh JH, Shin HJ, Kim SH, Kim JS, Kim BS. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells Dev. 2007. 16:421–428.

18. Lee CC, Ye F, Tarantal AF. Comparison of growth and differentiation of fetal and adult rhesus monkey. Stem Cells Dev. 2006. 15:209–220.
19. Battula VL, Bareiss PM, Treml S, Conrad S, Albert I, Hojak S, Abele H, Schewe B, Just L, Skutella T, Buhring HJ. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007. 75:279–291.

20. Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, Vukicevic S, Locatelli F, Fibbe WE. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res. 2007. 48:132–140.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download