Abstract
Hypoxic pulmonary vasoconstriction (HPV), a unique response of pulmonary circulation, is critical to prevent hypoxemia under local hypoventilation. Hypoxic inhibition of K+ channel is known as an important O2-sensing mechanism in HPV. Carbon monoxide (CO) is suggested as a positive regulator of Ca2+-activated K+ channel (BKCa), a stimulator of guanylate cyclase, and an O2-mimetic agent in heme moiety-dependent O2 sensing mechanisms. Here we compared the effects of CO on the HPV (Po2, 3%) in isolated pulmonary artery (HPVPA) and in blood-perfused/ventilated lungs (HPVlung) of rats. A pretreatment with CO (3%) abolished the HPVPA in a reversible manner. The inhibition of HPVPA was completely reversed by 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ), a guanylate cyclase inhibitor. In contrast, the HPVlung was only partly decreased by CO. Moreover, the partial inhibition of HPVlung by CO was affected neither by the pretreatment with ODQ nor by NO synthase inhibitor (L-NAME). The CO-induced inhibitions of HPVPA and HPVlung were commonly unaffected by tetraethylammonium (TEA, 2 mM), a blocker of BKCa. As a whole, CO inhibits HPVPA via activating guanylate cyclase. The inconsistent effects of ODQ on HPVPA and HPVlung suggest that ODQ may lose its sGC inhibitory action when applied to the blood-containing perfusate.
Pulmonary arteries show unique contractile response to hypoxia. The hypoxic pulmonary vasoconstriction (HPV) is physiologically and clinically important phenomenon to prevent systemic hypoxemia by diverting pulmonary blood flow from unventilated area to normoxic regions of lung. The oxygen (O2) sensing mechanisms of pulmonary arteries have been long dispute. Although the O2-dependent regulations of ion channels, especially the hypoxic inhibition of K+ channel, are strong candidate mechanisms, it is still unclear whether ion channels by themselves are the O2 sensing molecule (1-3).
TWIK-related acid-sensing K+ channel (TASK) is a member of two-pore domain K+ channel family, and has been suggested as a candidate of O2-sensing K+ channels in various types of tissues such as carotid body and pulmonary arterial smooth muscle (4). The hypoxic inhibition of TASK might explain, at least partly, the depolarization of pulmonary arterial smooth muscle and contractile responses under hypoxia (5). Recently, we found that the hypoxic inhibition of TASK-1 channel is mediated by NADPH oxidase type 4 (NOX-4), which is mediated by the heme moiety and heme-binding domains of NOX4 (6). In that study, it was found that the hypoxic inhibition of TASK-1 was prevented by a pretreatment with carbon monoxide (CO), which implicated that a high-affinity binding of CO with hemoprotein (NOX-4) might have mimicked the O2-binding state in spite of the hypoxic conditions.
CO has long been considered a toxic byproduct of incomplete combustion of coals and fuels. The toxic effect of CO is due to its strong affinity for hemoglobin and resultant impairment of O2 delivery by erythrocytes. In contrast to this conventional idea, an endogenous production of CO from heme metabolism by heme oxygenase (HO) has become well recognized. The endogenously produced CO is believed to play physiological roles such as anti-inflammatory and anti-apoptotic signals (7). In this regard, an exogenous application of CO at sub-lethal concentration has been suggested as a therapeutic maneuver to improve tissue implantation and to prevent vascular remodeling such as atheroscleoris (8). A recent animal study demonstrated that sublethal treatment with CO effectively prevents the medial proliferation (vascular remodeling) and pulmonary hypertension under chronic hypoxia (9). Such physiological and pharmacological effects of CO are thought to be mediated by multiple mechanisms such as soluble guanylate cyclase (sGC)-dependent production of cGMP and regulation of MAPK-pathways (10). In addition, CO has been also suggested as a positive regulator of large-conductance Ca2+-activated K+ channel (BKCa) via heme moieties that are associated with BKCa (11). The activation of BKCa by CO could induce hyperpolarization and relaxation of arteries (12).
Regarding to the regulation of putatively O2-sensing K+ channels by CO, it was tempting to investigate the physiological and pharmacological roles of CO in HPV and pulmonary arteries. The effects of CO on pulmonary blood flow and HPV have been previously investigated using perfused/ventilated lungs (V/P lungs) (13-16). In these studies, however, the exogenous application of CO showed diverse results; from essentially no effect to substantial inhibition of HPV depending on the tested ranges of CO concentrations (200 ppm to 12% of ventilated gas). In our pilot study using isolated pulmonary artery (PA), we found that CO treatment effectively abolish the contractile response of PA to hypoxia. From these backgrounds, we attempted to further investigate the effects of CO on the HPV of PA and V/P lungs. Our present study revealed intriguing difference between the HPV responses of PA (HPVPA) and lung (HPVlung) in terms of their modulation by exogenous CO.
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Seoul National University (IACUC approval no.: SNU-081209-1). Male Sprague-Dawley rats (250??00 g) were fully anaesthetized with pentobarbital sodium (40 mg/kg). A tracheotomy was performed, and the animals were ventilated with gas mixture of 21% O2 and 5% CO2 with balance made up by N2 with a Harvard respirator (small animal ventilator 683, Harvard Apparatus, Holliston, MA, USA). Stable ventilation (85 breaths/min) with regular tidal volume (10 mL/kg) was maintained. After a median sternotomy, lungs were exposed, and a suture was placed around the ascending aorta and the main pulmonary artery. After injection of heparin (200 U/kg) into the right ventricle, a cannula was inserted into the pulmonary artery via the right ventriculotomy, and the suture was tightened. Another cannula was into the left atrium via the left ventriculotomy and tied off the left ventricle, and then the blood was allowed to flow from the lung into the reservoir (37℃). The anesthetized rats were sacrificed during the above procedure by diverting blood flow to extra-corporal circulation. Lungs were perfused at a constant flow of 15 mL/min using a peristaltic pump (Servo amplifier 2990, Harvard Apparatus). The perfusate consisted of 20 mL of whole blood and 30 mL of physiological salt solution (PSS) resulting 10-15% hematocrit in 50 mL of the recycling perfusate. The mean PAP was measured using membrane pressure transducers connected to a side port of the inflow cannula, and the data acquisition and storage was done with Powerlab/4ST and Chart5 (ADInstruments). After a stabilization period, angiotensin II (1 µg) was injected into the circuit near the lung and then restabilization of pulmonary arterial pressure, the lungs were exposed to cycles of normoxia (5 min) and hypoxia (5 min) through ventilation with gas containing 21% or 3% O2 and 5% CO2 with balance made up by N2.
Lungs were rapidly removed from the fully anesthetized rats (see above), and the second- and third-order branches of PAs (I.D. 300-400 µm) were carefully dissected and cut into segments (2 mm in length). Segment of artery was mounted on two 40-µm wires in a Mulvany-type myograph (Myo-Interface Model 410A, DMT, Denmark). After a stabilization period in PSS continuously gassed with 74% N2, 21% O2 and 5% CO2 at 37℃, a basal tone (~0.5 g) was applied. Before the experiments, we evaluated viability of arteries using 80 mM KCl-PSS (equimolar substitution with NaCl). The bath solution was directly bubbled with hypoxic gas to lower the dissolved O2. Before the application of hypoxic gas, a low concentration of thromboxane A2 analogue (10 nM U46619) was added to bath solution, which induced a partial contraction. The U46619-induced pretone was equivalent with 5 to 10% of 80K contraction, and this partial contraction was necessary to evoke HPV in the isolated PAs in our experimental condition. We also measured the partial pressure of O2 (Po2) nearby the PA using a micro-oxygen electrode (MI-730, Microelectrodes Inc., Bedford, NH, USA), and confirmed that the Po2 was dropped to 3-4% by bubbling with the hypoxic gas (>5 min).
The PSS used in ventilated/perfused lung experiment consisted of the following composition (in mM): NaCl 131, KH2PO4 1.2, NaHCO3 22.6, CaCl2 3.2, MgSO4 1.2, glucose 11 and Albumin 30.0 g/L. The PSS used in myograph experiment with PA consisted of the following composition (in mM): NaCl 118, KCl 4, NaH2PO4 0.44, NaHCO3 24, CaCl2 1.8, MgSO4 1 and Glucose 5.6. Isotonic high K+ solutions (80 mM) were prepared by replacing NaCl with an equimolar amount of KCl in PSS solution. Bovine serum albumin (BSA) 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ) was purchased from Tocris (Ellisville, MO, USA). Zinc protoporphyrin IX (ZnPP) was obtained from Sigma (St. Louis, MO, USA). ZnPP was dissolved in 0.1 N NaOH, titrated to pH 7.4 with 0.1M HCl and diluted with phosphate buffered solution (PBS).
The data is presented as the original recordings and bar graphs of the mean±SEM (for n tested animals and arteries). Where necessary, One-way analysis of variance (ANOVA) and Bonferoni multiple range tests were used for the statistical analysis. P value <0.05 was considered significant.
The isometric tension of PA was measured under continuous aeration (21% O2/5% CO2). For normalization, high K+ (80 mM)-induced contraction (80K contraction) was confirmed in each vessel. After returning to control solution, 10 nM U46619 (Thromboxane A2 stable analogue) was applied to induce a 'pretone' that was equivalent with 5 to 10% of 80K contraction. In the pretone state, a robust HPV was consistently observed by bubbling with hypoxic gas (Po2, 3%, 91±6.1% of 80K contraction, n=7, Fig. 1A). Application of 3% CO in normoxic condition did not affect the pretone induced by U46619, while completely blocked the HPVPA (Fig. 1B). The suppressed HPVPA was completely reversed to 105±7.9% of 80K contraction by CO washout (n=8, Fig. 1B).
In a previous study of coronary arteries, the activity of BKCa is known to be increased by CO (12). Therefore, we tested whether the application of 2 mM TEA, a potent blocker of BKCa, recover the HPV under the inhibition by CO. However, the tone of PA was only slightly increased by TEA, and the HPVPA was recovered only after the removal of CO (Fig. 2A, n=3). Next, we tested whether the guanylate cyclase is involved in the inhibition of HPVPA by CO. The application of ODQ (30 µM), an inhibitor of soluble guanylate cyclase, effectively recovered the HPVPA (Fig. 2B, n=3).
The above results suggested that an activation of sGC by CO and cGMP-dependent signaling effectively inhibited the HPVPA. Then we tested the effects of CO on the HPVlung. In the V/P lungs of rats, an increase of PAP in response to hypoxic (Po2, 3%, 5 min) ventilation (ΔPAPhypox) was measured to monitor the HPV. The repetitive hypoxic ventilation with 5 min of recovery time in normoxic ventilation showed similar amplitudes of ΔPAPhypox. After confirming the consistent ΔPAPhypox, CO (0.3, 1, and 3%) was applied before the subsequent challenge of hypoxia. The amplitude of ΔPAPhypox was decreased by CO in a dose-dependent manner (Fig. 3, n=5). The basal PAP was also decreased by CO at 3%. However, the inhibition of HPVlung was incomplete even at 3% CO. The partial inhibitory effects of CO on the HPVlung and basal PAP were reversed by ventilating with CO-free gases. In addition, the removal of CO revealed a kind of rebound increase of basal PAP and HPVlung (Fig. 3A).
The partial inhibition of HPVlung by CO was not affected by blocking BKCa using 2 mM TEA (Fig. 4A, n=6). However, different from the response of HPVPA, the partial inhibition of HPVlung was also unaffected by ODQ (Fig. 4B, n=5). Interestingly, in the presence of 10 µM ODQ, an application of 10 µM L-NAME still induced a marked increase in basal PAP and ΔPAPhypox. Furthermore, an additive CO application decreased the amplitude of ΔPAPhypox to a similar extent as in control (Fig. 4C, n=2). The ineffectiveness might indicate that ODQ could not inhibit sGC by unknown reasons when applied to the blood-containing perfusate. Otherwise, CO inhibits HPVlung in sGC-independent manner (see Discussion).
Finally, we tested whether HPVlung is regulated by endogenously produced CO via HO-dependent pathway. A pretreatment of 30 µM ZnPP, an inhibitor of HO, appeared to induce a slight increase in HPVlung (Fig. 5, n=3, 119±9.2% of control HPV). However, the statistical significance could not be determined.
In this study, an exogenous application of 3% CO completely inhibits the HPVPA whereas the same level of CO had only a partial inhibitory effect on HPVlung in the rats. The test of ODQ on these differential responses revealed more perplexing results; the HPVPA was completely recovered from the CO-induced inhibition whereas the partially inhibited HPVlung was unaffected by ODQ treatment. A previous study by other researchers also showed partial inhibition of HPVlung by CO, which is unaffected by the pretreatment with NOS inhibitor (16). Our present results in V/P lung model (insignificant effects of L-NAME and ODQ) are consistent with this report in terms of cGMP-independent inhibition of HPV by CO. However, as mentioned above, the recovery of HPVPA by ODQ was inconsistent with these findings, for which we do not have a satisfactory explanation yet. Also, the ineffectiveness of HO inhibitor on the HPVPA and on the basal tone of PAs indicates that the putative role of endogenous CO by HO is less likely in the O2 sensing mechanisms of PA.
If ODQ had actually inhibited sGC in the V/P lungs, it was expected that the application of ODQ alone would mimic the effects of L-NAME because the vasorelaxation by endogenous NO is mainly mediated by cGMP. However, in contrast to the prominent increase of basal PAP and HPVlung by L-NAME, the ODQ pretreatment alone did not change these parameters (Fig. 4). Moreover, in the ODQ-pretreated V/P lungs, the additive application of L-NAME showed increased basal PAP and HPVlung responses. From these results, it was suggested that ODQ applied to the blood containing perfusate might somehow lose its inhibitory action on sGC in V/P lungs. While the concentration of ODQ (30 µM) used in this study was much higher than the reported IC50 on sGC (40??00 nM) (17), we could not exclude such possibility.
Interestingly, an earlier study of ODQ showed that the potent inhibition of sGC by ODQ is not directly associated with the prevention of the NO donor-induced relaxation in bovine PAs in that the anti-relaxing effect requires much higher concentrations (1-10 µM) of ODQ (17). In this respect, the inhibition of HPVPA by CO and its recovery by ODQ might be also cGMP-independent events. Such speculation is actually consistent with the conclusion of previous studies in V/P lungs (16). Lastly, the more prominent PAP increase by L-NAME than by ODQ might suggest multiple vasodilatory mechanisms of NO; sGC-independent relaxing effects of NO (18, 19).
As mentioned in Introduction, the present study was initially triggered by our previous experimental finding; prevention of hypoxic TASK-1 inhibition by CO (6). In this study, the heme-binding domain of NOX4 has been proposed as the O2 sensing module that is also affected by CO. Since a deletion of NADPH-binding domain did not affect the NOX4-mediated TASK-1 regulation by O2, it was suggested that a non-enzymatic actions of NOX4 was suggested as the mechanism for hypoxic inhibition of TASK-1 (6). While not shown in our present study, the application of anandamide, an inhibitor of TASK-1 (4), did not induce a significant contraction of PAs (data not shown). Therefore, the hypoxic inhibition of TASK-1 might not play a major role of HPV in rats. Also, the complete recovery of HPVPA by ODQ did not support the role of NOX4-like O2 sensor molecules in HPV.
In general, the consistent contractile response of isolated PA to hypoxia has been used as an experimental model to investigate the cellular mechanisms of HPV. The differential sensitivities of HPVlung and HPVPA to 3% CO indicated that the underlying mechanisms of HPV are more complex in V/P lungs than isolated PAs. The vasoactive substances and neurotransmitters released from lung parenchyma and blood cells might have additive or synergistic effects on the hypoxic responses, and thereby showed persistent HPVlung under CO treatment. While the natures of facilitating factor are still incompletely known, our previous study showed that the blood cells are critical for maintaining the HPVlung (20). The high affinity binding of CO with hemoglobin might also affect the reactivity of PA in V/P lungs. However, since the inhibition of HPV by CO was readily reversible by CO-free ventilation or bubbling, the involvement of hemoglobin is a less-likely explanation. Another concern was that the CO concentration in the V/P lung might not reached an equilibrium due to the high affinity of hemoglobin to CO. However, a previous in vivo study of rats showed that the plasma CO concentration readily reaches steady-state equilibrium within 5 min when ventilated with 1% CO (21). In our present study, the decrease of HPVlung by prolonged CO ventilation was not different from the acute response to CO (see Fig. 3 and 4). As a whole, however, the results of studies on HPV mechanism require cautious interpretation in terms of experimental modes and conditions.
In summary, our study reveals intriguing differences in the sensitivity of HPV to exogenous CO; total vs partial inhibition in PA and V/P lungs, respectively. The resistance of HPVlung to CO implies redundant O2-sensing mechanisms in vivo. CO inhibits HPVPA via activating guanylate cyclase. The inconsistent effects of ODQ on HPVPA and HPVlung suggest that ODQ may lose its sGC inhibitory action when applied to the blood-containing perfusate. The requirements of relatively high concentration of CO to induce a partial inhibition of HPVlung indicate that the therapeutic application of CO (<300 ppm) in some previous reports (7-10) is not relevant with the mechanisms in HPV. Further investigation is required to understand the effects of an endogenous/exogenous CO on the chronic hypoxia-induced changes in cells and tissues (22).
Figures and Tables
Fig. 1
Abolishment of HPVPA by exogenous CO. (A) After confirming maximum contraction of PA by 80 mM KCl (80 K), 10 nM U46619 was applied as a pretone agent to induce a partial contraction. In the presence of U46619, hypoxia (Po2 3%) increased the tone of PA similar to the level of 80 K contraction. (B) CO pretreatment almost completely abolished the HPV in a reversible manner. Isometric tone of PA is normalized to the 80 K contraction, and averaged values are shown as bar graphs (mean±SEM) in right panels.

Fig. 2
Effects of TEA and ODQ on the CO-induced inhibition of HPVPA. (A) Under the inhibition of HPVPA by 3% CO treatment, an additive application of 2 mM TEA induced a partial increase of basal tone. Only after the removal of CO, a full HPVPA was observed. (B) Under the inhibition of HPVPA by 3% CO treatment, an additive application of 30 µM ODQ induced a strong contraction that was equivalent to the full HPVPA. Isometric tone of PA is normalized to the 80 K contraction, and averaged values are shown as bar graphs (mean±SEM) in right panels.
*P<0.05; †P<0.01.
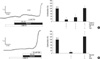
Fig. 3
Partial inhibition of HPV in V/P lungs by CO applied to the ventilating gas. (A) A representative trace of pulmonary artery pressure (PAP) and its increase by hypoxic ventilation (HPVlung). Both basal PAP and HPVlung are decreased by CO in a dose-dependent manner (0.3%, 1% and 3%). (B) Summaries of the HPVlung normalized to the initial control are shown as a bar graph (mean±SEM).
*P<0.05.

Fig. 4
Effects of ODQ, TEA and L-NAME on the HPV in V/P lungs. (A, C) The pretreatment with ODQ did not prevent the inhibition of HPVlung by CO. (B) The partial inhibition of HPVlung is also unaffected by 2 mM TEA. The treatment with TEA alone increases the basal PAP and HPVlung. Summaries of the HPVlung normalized to the initial control (%) are shown as bar graphs (mean±SEM, right panels). *P<0.05; †P<0.01. (C) The inhibition of HPV by CO is observed after the treatment with ODQ and 10 µM L-NAME. Note the large increase of basal PAP and HPVlung.

ACKNOWLEDGMENTS
We appreciate the technical advice by Prof. In-Ja Lim in Chuang-Ang University College of Medicine.
Notes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (no.A080190), the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (no.20090084124) and also by the Program for Basic-Clinical Medicine Collaborative Research, Seoul National University College of Medicine.
References
1. Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gudermann T, Schulz R, Seeger W, Grimminger F, Weissmann N. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J. 2008. 32:1639–1651.

2. Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006. 570:53–58.

3. Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol. 2005. 98:415–420.

4. Duprat F, Lauritzen I, Patel A, Honoré E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007. 30:573–580.

5. Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, Wilhelm J, Morty RE, Brau ME, Weir EK, Kwapiszewska G, Klepetko W, Seeger W, Olschewski H. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res. 2006. 98:1072–1080.

6. Park SJ, Chun YS, Park KS, Kim SJ, Choi SO, Kim HL, Park JW. Identification of subdomains in NADPH oxidase-4 critical for the oxygen-dependent regulation of TASK-1 K+ channels. Am J Physiol Cell Physiol. 2009. 297:C855–C864.
7. Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006. 10:672–686.

9. Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006. 203:2109–2119.

10. Ryter SW, Morse D, Choi AM. Carbon monoxide: to boldly go where NO has gone before. Sci STKE. 2004. 230:RE6.

11. Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004. 306:2093–2097.

12. Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005. 97:805–812.
13. Vassalli F, Pierre S, Julien V, Bouckaert Y, Brimioulle S, Naeije R. Inhibition of hypoxic pulmonary vasoconstriction by carbon monoxide in dogs. Crit Care Med. 2001. 29:359–366.

14. Zhang F, Kaide JI, Yang L, Jiang H, Quan S, Kemp R, Gong W, Balazy M, Abraham NG, Nasjletti A. CO modulates pulmonary vascular response to acute hypoxia: relation to endothelin. Am J Physiol Heart Circ Physiol. 2004. 286:H137–H144.

15. Miller MA, Hales CA. Role of cytochrome P-450 in alveolar hypoxic pulmonary vasoconstriction in dogs. J Clin Invest. 1979. 64:666–673.

16. Tamayo L, Lopez-Lopez JR, Castaneda J, Gonzalez C. Carbon monoxide inhibits hypoxic pulmonary vasoconstriction in rats by a cGMP-independent mechanism. Pflugers Arch. 1997. 434:698–704.

17. Brunner F, Stessel H, Kukovetz WR. Novel guanylyl cyclase inhibitor, ODQ reveals role of nitric oxide, but not of cyclic GMP in endothelin-1 secretion. FEBS Lett. 1995. 376:262–266.

18. Mingone CJ, Gupte SA, Quan S, Abraham NG, Wolin MS. Influence of heme and heme oxygenase-1 transfection of pulmonary microvascular endothelium on oxidant generation and cGMP. Exp Biol Med (Maywood). 2003. 228:535–539.

19. Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2009. 47:93–107.

20. Baek EB, Yoo HY, Park SJ, Kim HS, Kim SD, Earm YE, Kim SJ. Luminal ATP-induced contraction of rabbit pulmonary arteries and role of purinoceptors in the regulation of pulmonary arterial pressure. Pflugers Arch. 2008. 457:281–291.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download