Abstract
Transfusion-related acute lung injury (TRALI) is a serious adverse transfusion reaction that is presented as acute hypoxemia and non-cardiogenic pulmonary edema, which develops during or within 6 hr of transfusion. Major pathogenesis of TRALI is known to be related with anti-HLA class I, anti-HLA class II, or anti-HNA in donor's plasma. However, anti-HLA or anti-HNA in recipient against transfused donor's leukocyte antigens also cause TRALI in minor pathogenesis and which comprises about 10% of TRALI. Published reports of TRALI are relatively rare in Korea. In our cases, both patients presented with dyspnea and hypoxemia during transfusion of packed red blood cells and showed findings of bilateral pulmonary infiltrations at chest radiography. Findings of patients' anti-HLA antibodies and recipients' HLA concordance indicate that minor pathogenesis may be not as infrequent as we'd expected before. In addition, second case showed that anti-HLA class II antibodies could be responsible for immunopathogenic mechanisms, alone.
Transfusion-related acute lung injury (TRALI) is typically presented as pulmonary edema with bilateral pulmonary infiltrations on chest radiography and severe dyspnea, tachypnea and cyanosis which develops during or within 6 hr of transfusion (1). The diagnostic criteria was proposed by TRALI Consensus Conference in 2004 (Table 1) (2). Most patients having suspected TRALI require supplemental oxygen and approximately 70% of affected patients require mechanical ventilation. Although the estimated incidence of TRALI is 1 in 5,000 and the mortality is 6%, the true frequency is not known (1, 3). Suspected cases of TRALI may have been misdiagnosed or underestimated until now due to the poor awareness or lack of laboratory evidence.
Many studies revealed that the mechanism of TRALI is triggered by the presence of anti-human leukocyte antigen (anti-HLA) or anti-human neutrophil antigen (anti-HNA) antibodies present in plasma of donor and/or recipient. The identification of anti-HLA or anti-HNA antibodies in plasma of donor and/or recipient supports the diagnosis of TRALI (2).
Although there are a few documented reports about TRALI in Korea, the identification of immunopathogenic evidence was not performed sufficiently (4). Recently, we've experienced two cases of TRALI triggered by patients' anti-HLA antibodies which were specific for HLAs of transfused packed red blood cells (PRBC). Clinical manifestations as well as the findings of donors' and patients' HLA and anti-HLA concordance strongly supported the diagnosis of TRALI triggered by minor pathogenesis.
A 66-yr-old man having lung cancer with fibrosis was admitted through emergency room due to dyspnea on August 25, 2008. The initial vital signs were blood pressure 97/56 mmHg, pulse rate 77/min, respiratory rate 22/min and body temperature 37.3℃ and laboratory findings were SpO2 80% and 3,960/µL-10.0 gm/dL-124×103/µL for white blood cells (WBC)-hemoglobin (Hb)-platelet, respectively. On the second hospital day, pancytopenia became aggravated (2,810/µL-7.8 gm/dL-72×103/µL for WBC-Hb-platelet, respectively), so transfusion of 2 units of PRBC was planned to correct anemia. He was transfused the first unit of PRBC without any adverse transfusion reactions. Approximately 5 min after starting transfusion of the second unit of PRBC, the patient presented dyspnea, cyanosis and shivering with elevation of body temperature (38℃), He developed severe respiratory failure (SaO2 <50%, PaCO2 25.7 mmHg, PaO2 25.8 mmHg, respiratory rate >60/min) and hypotension (systolic blood pressure <70 mmHg). Intubation and mechanical ventilation were required to maintain adequate oxygenation. The chest radiograpy taken after intubation showed interval aggravation of bilateral diffuse ground glass opacity of lung parenchyma with pleural effusion but with normal cardiothoracic ratio (Fig. 1B).
The echocardiogram taken soon after the patient was transferred to the intensive care unit showed normal LV cavity size and moderate LV systolic dysfunction (LV ejection fraction was 39%) with mild aortic regurgitation. And there was no evidence of pulmonary embolism or acute myocardial infarction. The patient did not have any clinical signs of transfusion associated-circulatory overload (TACO) such as jugular venous distension, systolic hypertension or gallop. B-type natriuretic peptide (BNP) (Triage, Biosite, United Kingdom) level measured an hour after the event of respiratory failure was 106 pg/mL which was slightly elavated (reference range 5-100 pg/mL). It has been suggested that if the level of BNP is higher than 250 pg/mL, it is indicative of congestive heart failure, but if less than 150 pg/mL, TRALI is more likely (5). Acute hemolytic transfusion reaction also has been excluded based on repeated compatible serologic cross-match result and absence of typical clinical features such as hemoglobinuria, decreased hemoglobin level or renal failure. One month ago, he had been transfused with 2 units of PRBC without any adverse transfusion reactions. Since there were no angioedema, wheezing, strigor or urticaria, the diagnosis of anaphylactic transfusion reaction was also excluded.
Patient's pre- and post-transfusion blood samples and remnants of 2 units of transfused PRBC were subjected to further immunologic studies. HLA typing and tests for panel reactive antibody (PRA) were performed by PCR-SSP (Biotest, Germany) and ELISA (GTI, USA), respectively. Prior to transfusion, the patient already had multiple anti-HLA antibodies (Anti-A32, Anti-DR8) which were specific for HLA type of PRBC (first PRBC: A*32/DR8 and second PRBC: A*33/CREGs). And the % PRAs of patient's serum after transfusion were further increased to 40% (Anti-A1, -A25, -A32, -B37, -B45, -B51, -B63) and 37% (Anti-DR8, -DR52) of anti-HLA class I and II antibodies, respectively. The spectrum of donor specific anti-HLA antibodies became broadened and the titers were elevated (Table 2).
The patient was managed under ventilatory support with inotropics. There was no clinical improvement in pulmonary complications and the patient could not wean ventilator support.
A 62-yr-old woman was hospitalized for palliative treatment of rectal cancer with multiple lung metastasis on October 1. 2008. She was under anti-hypertensive medications for 10 yr. On the 9th hospital day, she complained of mild dyspnea and had a radiological finding compatible with mild pulmonary edema (Fig. 2A). On the 13th hospital day, transfusion of 8 units of platelet concentrates (PC) and 2 units of PRBC were planned to correct thrombocytopenia and anemia (7,880/µL-8.0 g/dL-9×103/µL for WBC-Hb-platelet, respectively). She had transfusion histories of PC and PRBC previously. 8 units of PC were transfused without any adverse transfusion reactions. However, after transfusion of approximately 30mL of the first unit of PRBC, the patient complained of difficulty in breathing. SpO2 dropped to 85%, so the transfusion was immediately discontinued. Since then, oxygen supplementation was required to maintain adequate oxygenation. A chest radiography taken shortly after the onset of symptoms showed aggravated bilateral lung infiltrations (Fig. 2B). Patient's blood pressure was increased to 164/92 mmHg from 114/60 mmHg, but it became normalized to 105/61 mmHg after 2 and half hours. To differentiate TACO, blood pressure and clinical signs were carefully monitored. Further echocardiogram or BNP blood test were not done. However, TACO was less likely for worsening the patient's pulmonary symptoms and signs when considering the facts that blood pressure was decreased to the baseline level shortly after stopping transfusion and pulmonary edema with dyspnea was not improved by diuretics therapy during hospitalization. Other clinical entities which cause acute respiratory distress such as acute hemolytic transfusion reactions and anaphylactic transfusion reactions have been excluded in the same manner with the first case.
Similarly with the first case, patient's serum after transfusion had multiple anti-HLA antibodies (36.5% PRA for HLA Class II) including anti-DR4 which was specific for HLA type of transfused PRBC (Table 2).
Based on the clinical and laboratory findings including the presence of patient's anti-HLA antibodies which potentially could have reacted with donor's leukocyte antigens, the diagnosis of TRALI that is responsible for worsening the patient's respiratory function which had pre-existing mild pulmonary edema has been made.
In 1983, Popovsky and Moore first identified "TRALI" as a distinct clinical entity (6). Recently TRALI has risen many concerns and awareness as the leading cause of transfusion-related mortality. TRALI is defined as acute hypoxemia and non-cardiogenic bilateral lung infiltrations, which presents as severe dyspnea, tachypnea and cyanosis, and develops during or within 6 hr after completion of transfusion (1). TRALI must be differentiated clinically from TACO and a variety of other conditions including acute hemolytic transfusion reactions, anaphylactic transfusion reactions, pulmonary embolism, acute myocardial infarction and bacterial contamination of the transfused product (7).
Like TRALI, TACO is increasingly being rediscovered as a common complication of transfusion. There are a lot of questions about the possibility of coexistence of TRALI and TACO and the difficulties in assigning transfusion-associated respiratory failure to a single cause, even when TACO can be demonstrated clearly (7, 8). TACO is defined by a combination of clinical signs such as post-transfusion hypertension, tachycardia, jugular vein distension, elevated central venous pressure and pulmonary artery occlusion pressure, pulmonary edema with cardiomegaly on chest radiography and the prompt response to diuretics or treatment of ischemia. BNP and N-terminal pro-BNP (NT-pro-BNP), cardiac neurohormones specifically being secreted from the ventricles in response to volume expansion, pressure overload or hypoxemia, have been reported as useful markers for diagnosis or exclusion of TACO (8). However, Li and colleagues recently found that natriuretic peptides have limited diagnostic value in a differential diagnosis of pulmonary edema after transfusion in the critically ill patients. Because BNP and NT-pro-BNP secretions were attributed to stimulatory effect of hypoxemia, as well as volume expansion and pressure overload and accuracy of BNP tests could be affected by the timing of the blood collection and other underlying diseases (5). Therefore, we can not totally depend on elevated BNP or NT-pro-BNP to differentiate TRALI from TACO in patients presenting dyspnea and hypoxemia after transfusion. In our cases, the possibility of coexistence of TRALI and TACO couldn't be excluded completely.
All plasma containing blood components have been associated with development of TRALI, including whole blood, PRBC, PC, fresh frozen plasma (FFP), and rarely, cryoprecipitate, IV immunoglobulin, and stem cell preparations. Plasma rich blood components, such as FFP and PC were known to be most commonly implicated in TRALI (9). It was reported that almost any blood component containing about 50ml or more of plasma could be implicated in TRALI. Recently, one report showed that estimated residual plasma volume as low as 10-20 mL which contains donor derived leukocyte antibodies might cause TRALI (10).
The proposed pathogenesis of TRALI are multifactorial. The anti-HLA and anti-HNA antibodies and biologically active lipids in stored cellular blood components have been described (11). Most cases of TRALI has been so far considered to be caused by anti-HLA class I, anti-HLA class II, or anti-HNA antibodies in the donor's plasma, which is called the major pathogenesis. Transfusion of donor's antibodies into a recipient who has the cognate antigens leads to neutrophil activation and release of oxidative substances that damage the pulmonary endothelium. This injury to pulmonary endothelium may cause an imbalance between the amount of fluid entering the alveoli and being actively removed and then results in severe pulmonary edema (1, 3).
However, fewer than 10% of TRALI cases involve the reverse mechanism in which the recipient has anti-HLA or anti-HNA antibodies against donor's leukocyte antigens (12). This minor pathogenesis supports the diagnosis of our two cases of TRALI. Due to relatively small number of leukocytes in the transfused blood components, it has been questioned whether TRALI can be caused by leukocyte antibodies in the patient's serum. The development of TRALI even with a relatively few leukocytes transfused in our cases might be explained by the activation of the recipient's own leukocytes either as a consequence of activating factors released by the small number of transfused leukocytes after antibody binding or by an abnormal binding of the recipient's alloantibodies to his/her own leukocytes. Leukocyte antibodies in the recipient reacting with leukocytes in the blood product have also been described in several studies (1, 13-15).
TRALI has traditionally been associated with the infusion of anti-HLA class I antibodies in transfused blood components. However, TRALI caused by anti-HLA class II antibodies without simultaneous presence of anti-HLA class I antibodies have rarely been described. In recent report, it has been suggested that anti-HLA class II antibodies play a critical role in initiating TRALI development (16). Proposed mechanism is that HLA class II-expressing cells, such as monocytes, may lead to the production of IL-1β, TNF-α and tissue factors and release of these cytokines may result in the secondary activation of neutrophils and/or endothelial cells leading to endothelial damage, capillary leak, and eventually TRALI (12, 17, 18). Our second case supports the role of anti-HLA class II alone as the cause of TRALI.
There has been international movement to reduce the risk of TRALI (11, 19). Most efforts are focusing on reducing of the use of plasma components from female donors. Since the donors of the implicated blood component in TRALI have been usually multiparous women who produced multiple antibodies against paternal leukocyte antigens during pregnancies. According to the 2008 Annual Serious Hazards of Transfusion (SHOT) report of UK, no case of TRALI due to FFP was reported in 2005, 2006 and 2007, following the introduction of preferential use of FFP from male donors since late 2003. In 2008, observed rates of TRALI remain lower than those in 2003-2004 when TRALI risk reduction strategies were first initiated and no deaths occurred as a result of TRALI which is the lowest rate since 1996. Base on these, SHOT organization emphasizes the need to achieve 100% use of male FFP and plasma for platelet pooling across the UK (20).
In conclusion, TRALI must be considered as a serious but not infrequent adverse transfusion reaction which needs to be diagnosed promptly in a ways of clinical suspicion, and radiological, biochemical and immunological findings and treated appropriately. Strategies and maximal efforts to prevent TRALI should be emphasized.
Figures and Tables
Fig. 1
Chest radiography of the Case 1. (A) 1 day before the transfusion of packed red blood cells (B) shortly after TRALI develops, showing aggravated bilateral lung infiltrations with pleural effusion.
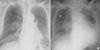
Fig. 2
Chest radiography of the Case 2. (A) 4 day before the transfusion of platelet concentrates and packed red blood cells, showing mild pulmonary edema (B) shortly after TRALI develops, showing aggravated bilateral lung infiltrations.
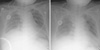
Table 1
Diagnostic criteria for TRALI and possible TRALI (TRALI Consensus Conference in Toronto, Canada, on April 1 and 2, 2004)

References
1. Danielson C, Benjamin RJ, Mangano MM, Mills CJ, Waxman DA. Pulmonary pathology of rapidly fatal transfusion-related acute lung injury reveals minimal evidence of diffuse alveolar damage or alveolar granulocyte infiltration. Transfusion. 2008. 48:2401–2408.

2. Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004. 44:1774–1789.

3. Middelburg RA, van Stein D, Briet E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: a systematic review. Transfusion. 2008. 48:2167–2176.

4. Huh JY, Han TH, Seo JW, Kim DC, Roh DH, Han KS. A case of transfusion-related acute lung injury. Korean J Blood Transfus. 2005. 16:250–254.
5. Li G, Daniels CE, Kojicic M, Krpata T, Wilson GA, Winters JL, Moore SB, Gajic O. The accuracy of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic) in the differentiation between transfusion-related acute lung injury and transfusion-related circulatory overload in the critically ill. Transfusion. 2009. 49:13–20.

6. Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983. 128:185–189.
7. Cherry T, Steciuk M, Reddy VV, Marques MB. Transfusion-related acute lung injury: past, present, and future. Am J Clin Pathol. 2008. 129:287–297.
8. Fiebig EW, Wu AH, Krombach J, Tang J, Nguyen KA, Toy P. Transfusion-related acute lung injury and transfusion-associated circulatory overload: mutually exclusive or coexisting entities? Transfusion. 2007. 47:171–172.

9. Win N, Massey E, Lucas G, Sage D, Brown C, Green A, Contreras M, Navarrete C. Ninety-six suspected transfusion related acute lung injury cases: investigation findings and clinical outcome. Hematology. 2007. 12:461–469.

10. Win N, Chapman CE, Bowles KM, Green A, Bradley S, Edmondson D, Wallis JP. How much residual plasma may cause TRALI? Transfus Med. 2008. 18:276–280.

11. Su L, Kamel H. How do we investigate and manage donors associated with a suspected case of transfusion-related acute lung injury. Transfusion. 2007. 47:1118–1124.

12. Curtis BR, McFarland JG. Mechanisms of transfusion-related acute lung injury (TRALI): anti-leukocyte antibodies. Crit Care Med. 2006. 34:5 Suppl. S118–S123.

13. Steciuk M, Marques MB. A new "initial" case of transfusion-related acute lung injury. Blood. 2008. 111:5257–5258.

14. Stroncek DF, Klein HG. Heavy breathing in the blood bank: is it transfusion-related acute lung injury, our anxiety, or both. Transfusion. 2007. 47:559–562.

15. Bux J, Becker F, Seeger W, Kilpatrick D, Chapman J, Waters A. Transfusion-related acute lung injury due to HLA-A2-specific antibodies in recipient and NB1-specific antibodies in donor blood. Br J Haematol. 1996. 93:707–713.

16. Nishimura M, Hashimoto S, Takanashi M, Okazaki H, Satake M, Nakajima K. Role of anti-human leucocyte antigen class II alloantibody and monocytes in development of transfusion-related acute lung injury. Transfus Med. 2007. 17:129–134.

17. Flesch BK, Neppert J. Transfusion-related acute lung injury caused by human leucocyte antigen class II antibody. Br J Haematol. 2002. 116:673–676.

18. Kopko PM, Paglieroni TG, Popovsky MA, Muto KN, MacKenzie MR, Holland PV. TRALI: correlation of antigen-antibody and monocyte activation in donor-recipient pairs. Transfusion. 2003. 43:177–184.

19. Sachs UJ. The pathogenesis of transfusion-related acute lung injury and how to avoid this serious adverse reaction of transfusion. Transfus Apher Sci. 2007. 37:273–282.

20. Cohen H, Taylor C, Mold D, Jones H. The 2008 Annual SHOT(Serious Hazards of Transfusion) Report. 2009. accessed on Nov. 15 2009. Available at
http:www.shot-uk.org.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download