Abstract
Glucocorticoid-remediable aldosteronism (GRA) is an autosomal-dominant inheritable form of hyperaldosteronism with early onset hypertension. GRA is caused by unequal crossing-over of the steroid 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) genes. As a result of chimeric gene duplication, aldosterone is ectopically synthesized in the adrenal zona fasciculata under the control of adrenocorticotropin. Here, we describe three cases of GRA in a Korean family. The proband-a 21-yr-old female-was incidentally found to have high blood pressure (170/108 mmHg). Her 46-yr-old father had been treated twice for cerebral hemorrhage at the ages of 29 and 39 yr. Her 15-yr-old brother had a 2-yr history of hypertension; however, he was never treated. Their laboratory test results showed normokalemia, hyporeninemia, hyperaldosteronism, and a high plasma aldosterone concentration-to-plasma renin activity ratio. Normal saline loading failed to suppress aldosterone secretion. However, dexamethasone administration effectively suppressed their plasma aldosterone concentrations. Following genetic analyses with PCR and direct sequencing to document the chimeric gene and crossover site, respectively, we identified CYP11B1/CYP11B2 and determined the breakpoint of unequal crossover to be located between intron 2 of CYP11B1 and exon 3 of CYP11B2.
Glucocorticoid-remediable aldosteronism (GRA) is an autosomal-dominant form of familial hyperaldosteronism associated with a high incidence of subarachnoid hemorrhages (1). This type of hyperaldosteronism results from unequal crossover of the genes encoding steroid 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2). The regulatory sequence of CYP11B1 and the coding sequence of CYP11B2 constitute chimeric gene CYP11B1/CYP11B2 (2). Therefore, the aldosterone synthase activity of CYP11B1/CYP11B2 is regulated by adrenocorticotropic hormone (ACTH) rather than the renin-angiotensin system. Normally, aldosterone synthase is expressed only in the adrenal glomerulosa, but this ACTH-responsive chimeric gene is ectopically expressed in the adrenal zona fasciculata, resulting in the production of aldosterone synthase and hybrid steroids such 18-oxosteroid and 18-hydroxysteroid (3).
The diagnosis of GRA is clinically important because it is treated specifically by suppressing ACTH with a synthetic glucocorticoid such as prednisolone. Although the diagnosis is supported by the dexamethasone suppression test, this test shows positive results for the other forms of familial hyperaldosteronism as well (4). Instead, a definitive diagnosis can be made by identifying the chimeric CYP11B1/CYP11B2 gene with either Southern blotting (2) or PCR (5).
In this report, we describe three cases of GRA in a Korean family. We performed genetic analyses by using PCR and direct sequencing to document the chimeric gene and crossover site, respectively.
A 21-yr-old female was referred to our clinics for further evaluation of thyroid incidentaloma in March 2009. On physical examination, her thyroid gland showed no positive findings, but her blood pressure was 170/108 mmHg. On studying the family history, we found that her father, aged 46 yr, had been treated twice for cerebral hemorrhage at the ages of 29 and 39 yr. Furthermore, her 15-yr-old brother also had a 2-yr history of hypertension but was never treated (Fig. 1). On laboratory workup, her routine laboratory and thyroid function tests were normal. Following ultrasound-guided fine-needle aspiration cytology for the 9-mm-sized thyroid incidentaloma, which showed hypoechogenicity, a diagnosis of adenomatous goiter was made. Under the impression of secondary hypertension, additional laboratory workup was conducted, showing the following results: plasma aldosterone concentration (PAC), 24.8 ng/dL (1-16 ng/dL); plasma renin activity (PRA), 0.45 ng/mL/h (0.20-2.70 ng/mL/h); PAC/PRA ratio, 55.1; sodium, 140 mEq/L (135-153 mEq/L); potassium, 4.0 mEq/L (3.5-5.3 mEq/L). To confirm hyperaldosteronism, normal saline loading was performed, but her PAC was not suppressed from 55.6 ng/dL to 52.6 ng/dL (Fig. 2A). Adrenal CT showed neither adrenal adenoma nor adrenal hyperplasia. On the basis of her family history, strongly suggesting familial hyperaldosteronism, we prescribed 2-mg dexamethasone for 2 days to the patient. As expected, dexamethasone treatment induced a significant decline in the PAC, from 84.7 ng/dL to 12.8 ng/dL (Fig. 2B). Taken together, we concluded that the most probable diagnosis is glucocorticoid-remediable hyperaldosteronism.
We decided to study her family to identify the chimeric gene. The proband's younger brother had higher blood pressure (204/116 mmHg) than her. His PAC was 42.2 ng/dL, PRA was 0.03 ng/mL/h, PAC/PRA ratio was 1407, sodium was 142 mEq/L, and potassium was 3.6 mEq/L; the liver parameters, blood cell counts, and renal parameters were normal. After normal saline loading, the PAC was not sufficiently suppressed although it decreased from 42.2 ng/dL to 24.2 ng/dL (Fig. 2C). Contrast-enhanced abdominal CT showed normal adrenal glands. After the dexamethasone suppression test, his PAC dramatically declined from 38.5 ng/dL to 1.1 ng/dL (Fig. 2D). Her father's blood pressure has been controlled in the range of 140/90 mmHg with antihypertensive medication prescribed by a local clinic since the diagnosis of cerebral hemorrhage at the age of 29.
After obtaining written informed consents from her family, we conducted the genetic analyses. However, because her mother and younger sister did not agree to this genetic analysis, we could not conduct it in these family members. Genomic DNA was extracted from anticoagulated blood specimens by using the QIAamp DNA Blood Mini Kit (Qiagen, Inc., Valencia, CA, USA). PCR for detecting the chimeric gene was performed by using a previously described method (5). DNA from the subjects produced a 4.0-kb PCR product when amplified with a pair of primers specific for CYP11B2. The CYP11B2 amplification for each individual served as a control for the integrity of each DNA sample. However, DNA from the subjects produced a 3.9-kb fragment when we used the sense primer for CYP11B1 and antisense primer for intron 5 of CYP11B2 during amplification (Fig. 3). After documenting chimeric gene CYP11B1/CYP11B2, we decided to perform sequence analysis of the chimeric PCR product to investigate the exact site of crossover between CYP11B1 and CYP11B2. As shown in Fig. 4, the breakpoint of unequal crossing-over was observed between intron 2 and exon 3 of the chimeric gene.
Following the diagnosis of GRA, magnetic resonance angiography (MRA) for detecting intracranial aneurysms was performed but showed no significant findings in the proband or her brother. Although her father's MRA result showed cystic encephalomalacia in the left basal ganglia and old infarctions in the left thalamus, left cerebellum, right basal ganglia, and right parietal lobe, presenting the sequelae of intracranial hemorrhage, he too had no intracranial aneurysm (data not shown).
The proband was prescribed 5-mg prednisolone daily. One month later, her blood pressure reduced to a normal level of 134/88 mmHg. Her brother was also prescribed 5-mg prednisolone daily, but his blood pressure did not show a marked decrease (from 170/108 mmHg to 164/86 mmHg). Therefore, we included spironolactone (50 mg) and lacidipine (4 mg) to his treatment regime.
Primary aldosteronism occurs in at least 5-9.5% of the cases of essential hypertension (4, 6), and the prevalence of GRA is thought to account for only 0.5-1.0% of the cases of primary aldosteronism (7). Actually, GRA is now considered to be the most common monogenic form of human hypertension (8). However, although there are many reports of patients with primary aldosteronism, we believe that this is the first report of a family with GRA in Korea.
GRA, also known as familial hyperaldosteronism type I, is an autosomal-dominant form of low-renin hypertension characterized by early onset of moderate-to-severe hypertension and a high incidence of premature cerebrovascular events (1). Patients with GRA show excessive production of aldosterone in their adrenal glands following ACTH stimulation and produce 18-oxygenated cortisols such as 18-hydroxycortisol and 18-oxocortisol, called hybrid steroids (3).
Although random screening of hypertensive individuals is ineffective for diagnosing GRA (9), hypertensive individuals with a strong family history of hypertension, especially those with a family history of cerebral hemorrhage, early onset hypertension, and multidrug-resistant hypertension, should be actively screened for GRA (8). For confirming primary hyperaldosteronism in patients suspected to have GRA, the dexamethasone suppression test is recommended as the next diagnostic step: an aldosterone level below 4 ng/dL strongly suggests GRA with high sensitivity (92%) and specificity (100%) (10). However, only 20% of the patients with dexamethasone-suppressible aldosteronism have chimeric gene CYP11B1/CYP11B2 (4). Therefore, a confirmatory diagnosis of GRA is possible only with genetic tests showing the chimeric gene.
Some authors have suggested that the crossover points of the chimeric gene differ between unrelated GRA families and that this difference affects the severity of hypertension (11). In our case, the crossover point was between intron 2 of CYP11B1 and exon 3 of CYP11B2. Although sequencing analysis of only one family with GRA cannot explain the role of the crossover points in the clinical severity, on the basis of a literature review (12), we consider that our sequencing analysis provides evidence suggesting that the proportion of CYP11B2 in the chimeric gene is responsible for inducing excessive aldosterone synthesis. Interestingly, the proband showed relatively lower blood pressure than her younger brother, and the hypertension was easily controllable with prednisolone. Actually, the phenotypic features of GRA can be diverse even within families and may be determined by various factors such as the proportion of CYP11B2 in the chimeric gene, sex, and other genetic traits such as mineralocorticoid receptor or sodium epithelial channel defects (13, 14). Taken together, the proband might be relatively protected by ovarian steroids or the genetic traits (15).
Administration of low-dose glucocorticoids such as prednisolone, suppressing pituitary ACTH secretion, is the treatment of choice for patients with GRA. The steroid dosage should be titrated against the blood pressure and maintained as low as possible to achieve adequate control. Individualized dosage titration does not result in normalization of biochemical markers such as urinary 18-oxosteroid or serum aldosterone levels, because these remain elevated in the majority of the patients with GRA whose blood pressure normalizes (15). If prednisolone does not effectively reduce the blood pressure, such as in the case of our proband's younger brother, type I mineralocorticoid receptor antagonists such as eplerenone and spironolactone or sodium epithelial channel antagonists such as amiloride and triamterene could be used successfully (16). However, nondirected antihypertensives such as β-blockers and angiotensin-converting enzyme inhibitors are less likely to be effective (17).
In summary, we have reported three cases of GRA in a Korean family, confirmed by genetic analyses. Although the dexamethasone suppression test is useful for diagnosis, detection of the chimeric gene is important for confirmatory diagnosis of GRA, for which PCR seems to be highly reliable. Therefore, hypertensive individuals with a strong family history of hypertension, especially those with a family history of cerebral hemorrhage, early onset hypertension, and multidrug-resistant hypertension, should be actively screened. If GRA is suspected, rigorous genetic analyses should be performed. These vigorous efforts to investigate the genetic traits of GRA family would make it possible to create guideline for GRA screening in Korea.
Figures and Tables
Fig. 1
The pedigree of the family showed with the proband indicated by black symbol.
Circles, females; squares, males; HTN, hypertension.
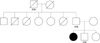
Fig. 2
Change of plasma renin activity (PRA) and plasma aldosterone concentration before and after normal saline loading test and dexamethasone suppression test. (A) Plasma renin activity (PRA) and plasma aldosterone concentration of proband before and after normal saline loading test. (B) Plasma renin activity (PRA), plasma aldosterone concentration and plasma cortisol of proband before and after dexamethasone suppression test. (C) Plasma renin activity (PRA) and plasma aldosterone concentration of younger brother of proband before and after normal saline loading test. (D) Plasma renin activity (PRA), plasma aldosterone concentration and plasma cortisol of younger brother of proband before and after dexamethasone suppression test.
Squares, plasma aldosterone concentration; triangle, plasma renin activity; circles, plasma cortisol.

Fig. 3
PCR analysis of DNA extracted from peripheral blood leukocytes of family members. Each subject was represented on the agarose gel. The left and right panels show aldosterone synthase and chimeric gene amplification, respectively.

Fig. 4
The breakpoints of "unequal crossing-over" by direct sequencing of PCR product. Nucleotide positions 1507 and 1508 of chimeric gene were identical to intron 2 of specific regions of CYP11B1. Nucleotide positions 46, 61 and 64 of chimeric gene exon 3 were identical with specific regions of CYP11B2. Each bold characteristic on nucleotide sequence of CYP11B1 or CYP11B2 is identical to corresponding nucleotide sequence of chimeric gene CYP11B1/CYP11B2.

References
1. Sutherland DJ, Ruse JL, Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J. 1966. 95:1109–1119.
2. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992. 355:262–265.
3. Mosso L, Gomez-Sanchez CE, Foecking MF, Fardella C. Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives. Hypertension. 2001. 38:688–691.

4. Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab. 2000. 85:1863–1867.

5. Jonsson JR, Klemm SA, Tunny TJ, Stowasser M, Gordon RD. A new genetic test for familial hyperaldosteronism type I aids in the detection of curable hypertension. Biochem Biophys Res Commun. 1995. 207:565–571.

6. Loh KC, Koay ES, Khaw MC, Emmanuel SC, Young WF Jr. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab. 2000. 85:2854–2859.

7. Pizzolo F, Trabetti E, Guarini P, Mulatero P, Ciacciarelli A, Blengio GS, Corrocher R, Olivieri O. Glucocorticoid remediable aldosteronism (GRA) screening in hypertensive patients from a primary care setting. J Hum Hypertens. 2005. 19:325–327.

8. McMahon GT, Dluhy RG. Glucocorticoid-remediable aldosteronism. Arq Bras Endocrinol Metabol. 2004. 48:682–686.

9. Gates LJ, Benjamin N, Haites NE, MacConnachie AA, McLay JS. Is random screening of value in detecting glucocorticoid-remediable aldosteronism within a hypertensive population? J Hum Hypertens. 2001. 15:173–176.

10. Litchfield WR, New MI, Coolidge C, Lifton RP, Dluhy RG. Evaluation of The dexamethasone suppression test for the diagnosis of glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 1997. 82:3570–3573.

11. Stowasser M, Gordon RD. Familial hyperaldosteronism. J Steroid Biochem Mol Biol. 2001. 78:215–229.

12. Stowasser M, Gunasekera TG, Gordon RD. Familial varieties of primary aldosteronism. Clin Exp Pharmacol Physiol. 2001. 28:1087–1090.

13. Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD. Severity of hypertension in familial hyperaldosteronism type I: relationship to gender and degree of biochemical disturbance. J Clin Endocrinol Metab. 2000. 85:2160–2166.

14. Dluhy RG, Lifton RP. Glucocorticoid-remediable aldosteronism (GRA): diagnosis, variability of phenotype and regulation of potassium homeostasis. Steroids. 1995. 60:48–51.

15. Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD. Treatment of familial hyperaldosteronism type I: only partial suppression of adrenocorticotropin required to correct hypertension. J Clin Endocrinol Metab. 2000. 85:3313–3318.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download