Abstract
This study attempted to assess the incidence and outcome of anthracycline cardiotoxicity and the role of dexrazoxane as a cardioprotectant in childhood solid tumors. The dexrazoxane group included 47 patients and the control group of historical cohort included 42. Dexrazoxane was given in the 10:1 ratio to doxorubicin. Fractional shortening and systolic and diastolic left ventricular diameters were used to assess the cardiac function. The median follow-ups were 54 months in the dexrazoxane group and 86 months in the control group. The mean cumulative doses of doxorubicin were 280.8±83.4 mg/m2 in the dexrazoxane group and 266.1±75.0 mg/m2 in the control group. The dexrazoxane group experienced significantly fewer cardiac events (27.7% vs. 52.4%) and less severe congestive heart failure (6.4% vs. 14.3%) than the control group. Thirteen cardiotoxicities including one cardiac death and 2 congestive heart failures occurred in the dexrazoxane group, and 22 cardiotoxicities including 2 cardiac deaths and 4 congestive heart failures, in the control group. Five year cardiac event free survival rates were 69.2% in the dexrazoxane group and 45.8% in the control group (P=0.04). Dexrazoxane reduces the incidence and severity of early and late anthracycline cardiotoxicity in childhood solid tumors.
Anthracyclines play an important role in combination chemotherapeutic regimens for both solid tumors and hematological malignancies in children (1, 2). However, anthracycline cardiotoxicity has been a major concern to pediatric oncologists and a limiting factor in prescribing anthracyclines (3, 4). The damage of the heart by anthracycline chemotherapy can be divided into an early and late cardiotoxicity. Early cardiotoxicity develops during treatment or in the first year after its completion, whereas late cardiotoxicity develops at least one year after therapy in cancer survivors (3, 4).
Although cumulative anthracyline doses are usually limited to 450-500 mg/m2 (5), a significant proportion of children treated with anthracycline even at doses less than 300 mg/m2 have evidence of cardiac dysfunction (6-8). Considering that the majority of long-term survivors of childhood cancers received an anthracycline during treatment, and that relatively high doses of anthracyclines remain essential to the clinical effectiveness of many chemotherapy regimens, cardioprotection from anthracycline is important in cancer therapy (3, 7).
An effective way to avoid the cardiotoxic effect of anthracyclines is to prevent cardiac injury during chemotherapy (9). To ameliorate the cardiotoxic side effect of anthracyclines, several strategies, including limitation of the cumulative dose of anthracyclines, different anthracycline dosage schedules, or different cardioprotective agents, have been studied (1, 8, 10). Currently, the most effective cardioprotectant for use in children is dexrazoxane (10, 11). This compound has been shown to offer a considerable protection from anthracycline cardiotoxicity in clinical studies of adults and children (10, 11). Although the results of the dexrazoxane studies in children are promising, evidence to make a recommendation for the use of dexrazoxane to prevent anthracycline cardiotoxicity in children is limited (10, 12).
This study attempted to assess the incidence and outcome of anthracycline cardiotoxicity and the role of dexrazoxane as a cardioprotectant in children with solid tumors, using serial echocardiography.
Since August 2001, dexrazoxane was introduced as a cardioprotective agent into the patients who had received anthracyclines. We enrolled patients who were diagnosed as having solid tumors and treated them in our institution with the same chemotherapeutic regimen from August 2001 to August 2005 (dexrazoxane group). Doxorubicin was administered at a dose of 30 mg/m2 in combination with cisplatin 60 mg/m2, cyclophosphamide 60 mg/kg, and etoposide 200 mg/m2 at intervals of 4 weeks with or without chest radiation therapy. Autologous peripheral blood stem cell transplantation (PBSCT) following high-dose chemotherapy consisted with melphalan 210 mg/m2, etoposide 800 mg/m2, and carboplatin 1,600 mg/m2 was indicated for high risk patients. Because it was not feasible to enroll a control group without cardioprotection at the same time, we recruited a historical control cohort (control group) treated with the same chemotherapy, including doxorubicin without dexrazoxane, in the period of January 1995 to July 2001. Supportive treatment, including hematopoietic growth factors, antibiotics, and transfusions, was given according to the guidelines of our institution without change over the period of patient enrollment.
Doxorubicin was administered intravenously as a bolus infusion. Dexrazoxane (Cardioxane, Chiron, The Netherlands) was administered intravenously 30 min prior to each dose of doxorubicin in the 10:1 ratio dexrazoxane:doxorubicin, as previously recommended (13, 14). There was no clinically significant side effect associated with dexrazoxane administration.
Cardiac function was assessed by echocardiogram in both groups. Echocardiographic parameters fractional shortening (FS), and systolic and diastolic left ventricular (LV) diameters, were used to assess cardiac function, and they were examined by the recommended standard approach from the M-mode record.
Each individual examination was performed by the same cardiologist. Echocardiograms were required before the initiation of doxorubicin therapy and performed every 2-3 doses of doxorubicin before cumulative dose of doxorubicin reached 300 mg/m2, and after then, performed with every dose of doxorubicin administration.
In the case of decreased FS or increased LV dimension, doxorubicin therapy was discontinued. Echocardiography is also performed regularly on a yearly basis after completion of the treatment.
A cardiac event was defined as the existence of at least one of the following three criteria: 1) increased LV diastolic diameter for the age; 2) increased LV systolic diameter for the age; or 3) FS less than 28% at any time point of doxorubicin treatment (15). For the age-related normal echocardiographic measurement range of LV dimension, we referred to 'the age-related echocardiographic measurement standard of normal subjects' developed by Henry et al. (16). Subclinical or asymptomatic cardiotoxicity was diagnosed on the basis of changes in the FS and LV diameters, and clinical cardiotoxiticy was based on the presence of heart failure symptoms.
Severity of heart failure was assessed and classified following three levels/grades: 1) death due to congestive heart failure (CHF); 2) CHF requiring medication; or 3) subclinical or asymptomatic heart failure.
Cardiac toxicity during or within one year after the doxorubicin treatment was defined as early cardiotoxicity and, later than that period, defined as late cardiotoxicity. Cardiac event free survival was defined as the time from the date of first treatment until the date a cardiac event was first noted.
The study was approved by the Institutional Review Board of Seoul National University Hospital (No. 0110-084-006). Since all subjects were under 18 yr of age, the subject's parents gave their written informed consent on behalf of their children, and the parents were present during the examination.
The results were presented as mean±standard deviation followed by range and median values in parentheses. The statistical analysis was based on the Student's t-test and chi-square test. Probabilities of event-free survival and overall survival were estimated by the method of Kaplan and Meier. The log-rank test was used to assess differences between the dexrazoxane group and the control group. Logistic-regression analysis was used to identify covariates associated with cardiotoxicity. A P value less than 0.05 was considered to be a significant change. The statistical program SPSS v15 was used for the purpose of statistical analyses.
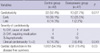
The dexrazoxane group included 47 patients (29 males, 18 females). Their diagnoses were 25 neuroblastoma, 4 peripheral primitive neuroectodermal tumors (PPNET), 15 retinoblastoma, and 3 other solid tumors. Three patients with cardiac dysfunction before doxorubicin therapy were excluded from the dexrazoxane group. The control group included 42 patients (25 males, 17 females). Their diagnoses were 40 neuroblastoma and 2 PPNET. Eleven patients were excluded from the historical cohort for the following reasons: one misdiagnosis, 2 incomplete records, 3 early withdrawals without echocardiographic evaluations, 3 congenital heart diseases, and 3 cardiac dysfunctions before doxorubicin therapy. Patients' characteristics were described in Table 1.
The median ages of the patients were 24 months (1-168) in the dexrazoxane group and 30 months (3-137) in the control group, which were not significantly different from each other (P=0.737). The number of patients received chest radiation therapy were 14 in the dexrazoxane group and 3 in the control group, which was significantly higher in the dexrazoxane group (P=0.007). The number of patients who underwent autologous PBSCT was 7 in the dexrazoxane group and 11 in the control group, which was not significantly different (P=0.966). The median follow-up durations were 54 months (7-93) for the dexrazoxane group and 86 months (7-158) for the control group, longer in the control group (P=0.008). The cumulative doses of doxorubicin were 280.8±83.4 mg/m2 (109-428; median 290) in the dexrazoxane group and 266.1±75.0 mg/m2 (87-388; median 294) in the control group, which were not significantly different (P=0.387) (Table 2).
In the dexrazoxane group, there were 12 early cardiotoxicities: in 7 patients during doxorubicin therapy, in 5 patients less than one year after doxorubicin therapy, and one late cardiotoxicity. In the control group, there were 16 early cardiotoxicities: in 12 patients during doxorubicin therapy, 4 patients less than one year after doxorubicin therapy, and 6 late cardiotoxicities (Table 3).
Most of the cardiotoxicity was subclinical and asymptomatic. In the dexrazoxane group, there were one cardiac death and 2 CHF requiring medication. In the control group, there were 2 cardiac deaths and 4 CHF requiring medication among 22 patients with cardiotoxicity.
Compared with the control group treated with doxorubicin alone, the dexrazoxane group treated with doxorubicin and dexrazoxane experienced significantly fewer cardiac events (52.4% vs. 27.7%, P=0.017) and a lower and less severe incidence of CHF (14.3% vs. 6.4%, P=0.049). The incidence of cardiac dysfunction in the disease free survivors was 19.4% (6/31) in the dexrazoxane group and 54.5% (12/22) in the control group, which showed a significant difference (P=0.03).
The 5 yr cardiac event free survival rates were 69.2% in the dexrazoxane group and 45.8% in the control group, which showed a significant difference (P=0.04) (Fig. 1). No case of secondary malignant neoplasm was observed during the follow-up period in both groups.
Logistic-regression analysis was used to determine which covariates were associated with decreased FS or increased LV dimension. Covariates were the treatment groups (use of dexrazoxane), sex (male vs. female), age (≤2 vs. >2 yr) at diagnosis, chest radiation therapy, hematopoietic stem cell transplantation, and cumulative doses of doxorubicin for early and late cardiotoxicity. Only use of dexrazoxane was associated with cardiotoxicity (P=0.017). Other covariates did not affect cardiotoxicity significantly. The cumulative doses of doxorubicin till cardiotoxicity were 286.1±66.5 mg/m2 (198-393; median 300) in the dexrazoxane group and 272.2±61.3 mg/m2 (120-380; median 299.5) in the control group, which were slightly higher in the dexrazoxane group, but not significantly different (P=0.539) (Table 2).
A clinical dilemma for pediatric oncologists is the need to balance the efficacy of anthracycline therapy against cardiotoxicity, which causes severe morbidity and reduced quality of life (10). Children and adolescents are particularly sensitive to the cardiotoxic effects of doxorubicin (5) because cardiac growth is impaired and is not able to keep face with the demands placed on the heart by normal somatic growth (1, 7). Risk of mortality from cardiac-related events is 8-times higher for long-term childhood cancer survivors than for the normal population (17).
There is a wide variation in the reported frequency of both clinical and subclinical cardiotoxicity after anthracycline therapy in children (10). Up to 70% of long-term survivors of childhood cancer have evidence of cardiac dysfunction, including overt CHF (5). The incidence of clinical heart failure has been reported to be as high as 16% 0.9 to 4.8 yr after treatment (18) and the incidence of subclinical heart failure at a median of 6.4 yr after treatment more than 57% (19). This variation was partially explained by different definitions of heart failure or different ways of assessing cardiac function (2).
Anthracycline cardiac damage presumably results from the formation of anthracycline-iron complexes within cardiac myocytes (2, 11). These complexes catalyze the formation of irondependent oxygen-free radicals that cause oxidative damage to the cells. Dexrazoxane is a bisdioxopiperazine compound acting by inhibiting topoisomerase II, scavenging free radicals, and chelating heavy metals (11). Dexrazoxane binds to intracellular irons and prevents the formation of free radicals. As a result, the myocardial free iron pool is reduced and iron is prevented from forming a complex with anthracyclines and causing cellular damage (11).
Dexrazoxane showed a statistically significant benefit in preventing heart damage, mostly in adults with advanced breast cancer (10, 20). No evidence for a difference in response rate or survival between the dexrazoxane and the control group was found. Dexrazoxane has not yet been introduced as a standard prophylaxis of cardiotoxicity in children because there is insufficient evidence to make a recommendation for the use of dexrazoxane in the treatment of pediatric malignancies (10, 12). The short-to-medium-term efficacy of dexrazoxane in preventing doxorubicin-induced cardiotoxicity was investigated in two randomized trials (5, 13) and several smaller, nonrandomized, controlled trials (9, 21, 22), including one studying different malignancies in children.
In this study, dexrazoxane reduced both the incidence and the severity of cardiotoxicity. The incidence of dose-limiting cardiac events was significantly lower in patients receiving dexrazoxane than in dexrazoxane-untreated controls. Thirteen cardiotoxicities (27.7%) occurred in the dexrazoxane group and 22 cardiotoxicities (52.4%) in the control group. Like other reports, most of the cardiotoxicities were subclinical and asymptomatic. Only 3 patients in the dexrazoxane group (6.4%) and 6 patients in the control group (14.3%) had clinical heart failure requiring medication or leading to death.
Despite the need for a high-quality randomized prospective study, it is not feasible to enroll large number of homogeneous patients during a short period and survey them for more than 10 yr. Therefore, we recruited historical controls who received the same chemotherapeutic regimen, including anthracycline without dexrazoxane, to compare with the study population treated with anthracycline and dexrazoxane.
Cardiotoxicity is reported to be related to the cumulative dose of anthracyclines (3, 8, 23) and, above 300 mg/m2, is known as a significant risk factor for decreased cardiac function (24). However, we could not find a definite dose-dependent increase of cardiotoxicity associated with cumulative doses of doxorubicin. The cumulative doses of doxorubicin were similar in both the dexrazoxane and the control group, even in patients with cardiotoxicities. Early cardiotoxicity seemed to increase with doxorubicin dose in the control group but decrease in the dose above 300 mg/m2 in the dexrazoxane group. It can be explained by the small numbers of patients and the management policy limiting additional doxorubicin in the case of abnormality on echocardiography.
In addition, the cumulative dose of doxorubicin until a cardiac event was relatively lower than reported and varied from 198 to 393 mg/m2 in the dexrazoxane group and 120 to 380 mg/m2 in the control group. This is consistent with the report by Lipshultz et al. (8) that found cardiac abnormalities in patients who received lower cumulative doses, even as low as 45 mg/m2. It suggests that there is no "safe dose" free from potential cardiac damage (8).
In this study, both early and late cardiac dysfunctions were defined as cardiac events. That is because most patients with early cardiotoxicity had persistent cardiac dysfunctions afterwards. The decline of FS and enlargement of the LV dimension tended to continue, even in the case of transient improvement in the course of follow-up. This agrees with the report that the degree of cardiac injury identified after the first dose of doxorubicin treatment is related to the likelihood of subsequent echocardiographic abnormalities (25). Cardiac abnormalities were persistent and progressive in the patients received anthracyclines for acute lymphoblastic leukemia (8).
All cancer survivors treated with anthracyclines should be screened for cardiac damage, symptomatic or not (26). However, the best way to monitor cardiac function during and after anthracycline treatment is controversial at present. Echocardiography is the most commonly used noninvasive method to measure LV function, but its sensitivity, specificity, and reproducibility are strongly influenced by interobserver variability (27). Other techniques used to determine cardiac injury include multigated radionuclide scans (MUGA); cardiac-specific biochemical markers, such as cardiac troponin T; natriuretic peptide; magnetic resonance imaging (MRI); and myocardial tissue biopsy (26). Lipshultz et al. (13) reported that their subjects showed only subclinical cardiotoxicity with elevated troponin T levels, and normal echocardiographic parameters in early phase of the treatment; however, in our study, abnormal echocardiographic reading was observed in many subjects in the early phase. One of the reasons of this difference could be that the ranges of normal/abnormal echocardiographic parameters used in the field were not unified. Therefore, an ideal method for cardiac function monitoring is necessary.
In alleviating cardiotoxicity, despite its clear cardioprotective effects, dexrazoxane is not routinely used because of concerns that it could diminish the anti-tumor activity for the anthracyclines or increase other chemotherapy-related toxicities, such as hematological or infectious complications (9, 10, 12). In meta-analysis of 9 randomized controlled trials, a lower response rate associated with dexrazoxane was not confirmed (10). Only for an abnormal white blood cell count at nadir showed significant difference compared to the control group (10). In our study, it was not feasible to compare overall survival rate which reflected the long-term efficacy of chemotherapy because of heterogeneity of diagnoses in the two groups. However, the number of patients who completed scheduled chemotherapy was higher in the dexrazoxane group, which reflected that toxicities limiting further chemotherapy were not prominent in the dexrazoxane group (data not shown).
The optimal dose ratio of dexrazoxane/anthracycline for cardioprotection has not yet been completely determined. The ratio of dexrazoxane to anthracycline dose varied between studies and was 6.25:1, 10:1 or 20:1 and all of the doses appeared equally well tolerated (10).
The possible higher risk of secondary malignant neoplasm associated with dexrazoxane administration in children with Hodgkin's disease (28) was not confirmed in the other randomized study in children with high risk acute lymphoblastic leukemia (29). In our study, there was no secondary malignant neoplasm in both the dexrazoxane group and the control group.
Cost effectiveness should be considered in the use of dexrazoxane as a routine management. The added cost of dexrazoxane would be justified considering the high cost required by serious sequelae, including congestive heart failure and even mortality from anthracycline-induced cardiomyopathy (30).
In addition to a cumulative dose of anthracyclines and length of post-therapy interval, young and advanced age, female sex, and radiation therapy involving the heart are considered to be risk factors for cardiotoxicity (17). None of these factors was associated with cardiotoxicity in this study, which is thought to be contributed by the smaller number of samples and limited follow-up periods. Concomitant drugs, including cyclophosphamide, rate of anthracycline administration, and type of anthracycline, were not considered in this study, because all patients were treated with the same chemotherapeutic regimen. This encouraging result, reflecting the benefit of cardioprotection needs to exclude the impact of a shorter follow-up time of 54 months in the dexrazoxane group.
In conclusion, dexrazoxane reduces the incidence and severity of early and late cardiotoxicity in children with solid tumors receiving doxorubicin chemotherapy. Administration of dexrazoxane was well tolerated and no second malignant neoplasm was observed during the follow-up period, which might be contributed by the limited follow-up period. This study supports the benefit of dexrazoxane as a cardioprotective agent in children who are vulnerable to cardiac damage by anthracycline. Evidently, more evidence from randomized controlled study and long-term follow-up more than 10 yr would be necessary to confirm the efficacy of dexrazoxane in children.
ACKNOWLEDGMENTS
The authors thank Mrs. Jonghee Y Shadix for her assistance in the preparation of this manuscript.
References
1. van Dalen EC, Caron HN, Kremer LC. Prevention of anthracycline-induced cardiotoxicity in children: the evidence. Eur J Cancer. 2007. 43:1134–1140.

2. van Dalen EC, Raphael MF, Caron HN, Kremer LC. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev. 2009. (1):CD006647.

3. Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997. 15:1544–1552.

4. Kwon HJ, Song YH, Kang SJ, Kang HJ, Choi HS, Bae EJ, Shin HY, Noh CI, Yun YS, Ahn HS. Follow-up study of children with anthracycline cardiotoxicity. J Korean Pediatr Soc. 2003. 46:242–249.
5. Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver-McClure L, Chen CC, Dilsizian V, Avila N, Jarosinski P, Balis FM, Poplack DG, Horowitz ME. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996. 14:362–372.

6. Sorensen K, Levitt G, Bull C, Chessells J, Sullivan I. Anthracycline dose in childhood acute lymphoblastic leukemia: issues of early survival versus late cardiotoxicity. J Clin Oncol. 1997. 15:61–68.

7. Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003. 97:1991–1998.
8. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005. 23:2629–2636.

9. Elbl L, Hrstkova H, Tomaskova I, Blazek B, Michalek J. Long-term serial echocardiographic examination of late anthracycline cardiotoxicity and its prevention by dexrazoxane in paediatric patients. Eur J Pediatr. 2005. 164:678–684.

10. van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2008. (2):CD003917.

11. Cvetkovic RS, Scott LJ. Dexrazoxane: a review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005. 65:1005–1024.
12. Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A 3rd, von Hoff D, Schuchter LM. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009. 27:127–145.

13. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004. 351:145–153.

14. Schuchter LM, Hensley ML, Meropol NJ, Winer EP. 2002 update of recommendations for the use of chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2002. 20:2895–2903.

15. Henry WL. Evaluation of ventricular function using two dimensional echocardiography. Am J Cardiol. 1982. 49:1319–1323.

16. Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980. 62:1054–1061.

17. Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME Jr, Ruccione K, Smithson WA, Robison LL. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001. 19:3163–3172.

18. Kremer LC, van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002. 13:503–512.

19. Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002. 13:819–829.

20. Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Dexrazoxane Study Group. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006. 17:614–622.

21. Elbl L, Hrstkova H, Tomaskova I, Michalek J. Late anthracycline cardiotoxicity protection by dexrazoxane (ICRF-187) in pediatric patients: echocardiographic follow-up. Support Care Cancer. 2006. 14:128–136.

22. Kovacs GT, Erlaky H, Toth K, Horváth E, Szabolcs J, Csoka M, Jokuti L, Erdelyi D, Muller J. Subacute cardiotoxicity caused by anthracycline therapy in children: can dexrazoxane prevent this effect? Eur J Pediatr. 2007. 166:1187–1188.

23. Nysom K, Holm K, Lipsitz SR, Mone SM, Colan SD, Orav EJ, Sallan SE, Olsen JH, Hertz H, Jacobsen JR, Lipshultz SE. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998. 16:545–550.

24. van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006. 42:3191–3198.

25. Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, Ottlinger ME. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997. 96:2641–2648.

26. Ruggiero A, Ridola V, Puma N, Molinari F, Coccia P, De Rosa G, Riccardi R. Anthracycline cardiotoxicity in childhood. Pediatr Hematol Oncol. 2008. 25:261–281.

27. Meinardi MT, van der Graaf WT, van Veldhuisen DJ, Gietema JA, de Vries EG, Sleijfer DT. Detection of anthracycline-induced cardiotoxicity. Cancer Treat Rev. 1999. 25:237–247.

28. Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007. 25:493–500.

29. Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, Clavell LA, Larsen EC, Moghrabi A, Samson Y, Schorin MA, Cohen HJ, Lipshultz SE, Sallan SE, Silverman LB. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008. 26:1106–1111.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download