INTRODUCTION
Our previous studies demonstrated that alterations in the renin angiotensin aldosterone system (RAAS), of the developing rat, affect cardiac and renal growth and development (1). To date, many studies have reported that RAAS plays a major role in the pathophysiology of the cardiovascular system (2, 3). Aldosterone, the final product of the RAAS, has been highlighted as a potentially important component of the RAAS. In the cardiovascular system, aldosterone modulates vascular tone by increasing the pressor response to catecholamines or by upregulation of angiotensin II receptors (4), and it provides a homeostastic response to hypovolemia (5). However, aldosterone excess causes collagen deposition and remodeling in vessels (5). Therefore, excessive aldosterone secretion promotes perivascular and cardiac fibrosis, myocyte hypertrophy and diastolic dysfunction that is independent of the other components of the RAAS by a variety of mechanisms (6).
The mineralocorticoid receptor antagonists, spironolactone and epelerenone, can prevent many of these deleterious effects, on the cardiovascular system. The mineralocorticoid receptor antagonist, spironolactone has been used to reverse these adverse effects on the cardiovascular system in many patients with chronic heart disease such as congestive heart failure or myocardial hypertrophy. Although several studies have investigated the role of aldosterone in the adult cardiovascular system (2, 3), relatively little attention has been focused on the role of aldosterone in modulating cardiac growth during the perinatal period. Aldosterone contributes to cardiomyocyte apoptosis both in vivo and in isolated cells (7). Apoptosis and cell proliferation are essential for normal cardiac development; dysregulation of this process can lead to a variety of cardiac diseases during postnatal development.
As a final product of the RAAS, aldosterone is now recognized as an important modulator of myocyte apoptosis (8). Among the mitogen-activated protein kinase (MAPK) family members, p38 is activated by hemodynamic stress and cellular stress; it is thought to inhibit cell growth and induce apoptosis. However, the extracellular signal-regulated kinase (ERK) promotes cell proliferation and differentiation (9). Clusterin and p53 are apoptosis related molecules. Clusterin plays a major role in preventing apoptosis and p53 may enhance the renin-angiotensin system and activates apoptosis (10). The transforming growth factor (TGF)-β is known as a multifunctional growth factor; it is involved in modulating cell proliferation, differentiation and extracellular matrix formation (11).
The intracellular molecular mechanisms involved in the control of cardiac growth and development, by aldosterone in vivo, have not been elucidated. Therefore, the present study was designed to investigate the role of endogenous aldosterone in cardiac growth and development, through apoptosis and cell proliferation, in the neonatal rat heart.
MATERIALS AND METHODS
Animal preparation
Forty-five neonatal rat pups from five pregnant Sprague Dawley rats were breastfed by their own mothers throughout the study. The body weights were measured daily from birth. The rat pups were given a dose of 200 mg/kg of spironolactone (Sigma Chemical Co., St. Louis, MO, USA) (S group) or normal saline (control group) through an orogastric tube. The dose of 200 mg/kg of spironolactone has been shown to block the effects on aldosterone (7).
This dose of spironolactone is known to protect cardiovascular system against the harmful effects of hemodynamic overload (12). The rats were sacrificed at 7 days of age. Their hearts were removed, weighed and harvested for this study. RNA analysis, protein assays, detection of apoptosis, and immunohistochemistry were performed. The Animal Care Committee of Korea University Guro Hospital approved the experimental protocol.
Immunohistochemical staining
For assessment of the expression of p38, p53, clusterin, and TGF β-2, the harvested hearts were prepared in 10% formalin solution (Sigma Chemical Co.) and embedded in paraffin. The samples were then cut into 4-µm sections and the sections were deparaffinized with xylene, followed by rehydration in a descending series of ethanol concentrations. The endogenous peroxidase was quenched by 3% hydrogen peroxide in methanol for 15 min. The sections were incubated with primary antibodies against p38 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p53 (Calbiochem, Cambridge, USA), clusterin (Upstate Biotechnology, Incorporated, NY, USA), and TGF β-2 (Promega, Madison, WI, USA). After the incubation, the sections were washed twice for 5 min in phosphate-buffered saline (PBS). The sections were then incubated for 30 min with secondary antibodies, washed in PBS and incubated for 50 min with Vectastain ABC reagent (Burlingame, CA, USA). The bound antibodies were detected using 3,3'-diaminobenzidine, which produces a brown color. The sections were counterstained with a 0.5% methyl green solution (Trevigen, Gaithersburg, MD, USA), then the samples were dehydrated and mounted. After dehydration, we evaluated the specimens under the light microscope.
Detection of cell proliferation
To assess cell proliferation, the harvested hearts were prepared in 10% formalin or Bouin solution (Sigma Chemical Co.), embedded in paraffin, then immunohistochemical staining for the proliferating cell nuclear antigen (PCNA) was carried out. The PCNA-positive cells were detected using the avidin-biotin immunoperoxidase method (Vectastain ABC kit). The primary antibodies were the monoclonal anti-mouse PCNA antibody (1:100 dilutions, DAKO, Glostrup, Denmark) in the study group and PBS in the negative control group. Each slide was cross-stained with 0.5% methyl green solution (Trevigen). After dehydration, we compared the slides under the light microscope. The PCNA positive cells were evaluated by counting 50 areas (25×25 µm) and the average was calculated. The counts were carried out randomly throughout all the observed fields.
Detection of apoptosis
To determine whether apoptosis is involved in the developing rat heart, we performed terminal deoxynucleotide transferase-mediated nick-end labeling (TUNEL) staining for apoptotic cells in the heart of the control and spironolactone-treated group. Apoptotic nuclei were labeled using the TACS TM 2 TdT In situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD, USA). Tissues were fixed in 4% neutral buffered formalin for 4 hr at 4℃, dehydrated in graded alcohols and embedded in paraffin. The samples were then cut into 4 µm sections and dried onto siliconized slides (Sigma Chemical Co.). The slides were deparaffinized and digested for 10 min with proteinase K (20 µg/mL) and the endogenous peroxidase activity was quenched by 2% hydrogen peroxide for 5 min. Samples were equilibrated using the provided buffer for 2 min and labeled with biotin dNTP mixtures, TdT, CoCl2, and a labeling buffer according to the manufacturer's instructions. The samples were rinsed in PBS for 2 min, and treated with the provided Streptavidin-Horseradish Peroxidase Detection solution for 10 min and finally washed 2 times in PBS for 2 min each wash. The color was developed using 0.05% 3,3'-diaminobenzidine and 0.05% hydrogen peroxidase in PBS for 5 min. The sections were rinsed in water and counter-stained in methyl green for 5 min, then the samples were dehydrated and mounted. After dehydration, we compared them under the light microscope. The positive apoptotic cells were counted in 50 areas (25×25 µm) and the average was calculated.
Isolation of RNA and analysis of mRNA
Five hearts from the control group and the spironolactone-treated group were selected for RNA analysis and protein assays. After removal from the rats, the cardiac tissue was frozen in liquid nitrogen and stored at -70℃. The total cellular RNA was isolated using the TRI-reagent (Molecular Research Center, Cincinnati, OH, USA) and homogenized with a tissue tearor (Model 985-370, Biospec products, Bartlesville, OK, USA). Next, 37% chloroform (200 µL/mL TRI reagent) was added to the homogenates and centrifuged at 12,000 rpm for 15 min at 4℃ separating the sample into three layers: RNA, DNA and protein material. The colorless clear upper layer was transferred into another Eppendorf tube, isopropanol was added, then left at room temperature for 15 min centrifuged at 12,000 rpm for 10 min at 4℃ until a white-colored cellular RNA pellet was isolated. The pellet was dried at room temperature for 5 min after washing in 75% ethanol and was dissolved in 25 µL Forma zol (Molecular Research Center) at 55℃ in a heating block for 10 min and then stored at -70℃. The RNA was quantified spectrophotometrically at an absorbance of 260 nm.
cDNA synthesis by reverse transcription (RT) and PCR
A cDNA Synthesis Kit (Boehringer Mannheim Corp., Indianapolis, IN, USA) was used to obtain 1 µg of oligo dT primed first strand cDNA from the RNA template. AMV reverse transcriptase was used for synthesis of the first strand cDNA for use in subsequent amplification reactions. The PCR reaction had a forward primer 5'-AATGCATCCTGCACCACCAA-3' and a reverse primer 5'-GTAGCCATATTCATTGTCATA-3' for the GAPDH designed based on the DNA template from rats. In addition, 5'-CGGGTACCGACAATGAGCTCCA-3', 5'-GGCCGCGGCCACTTCTGCAGAC-3' for clusterin, 5'-ATGTCTCAGGAGAGGCCCACGTTCT-3', 5'-TCAGGAGTCCATTTCTTCTTGGTC-3' for p38, 5'-CTGAGGTCGGCTCCGACTATACCACTATCC-3', 5'-CTGATTCAGCTCTCGGAACATCTCGAAGCG-3' for p53, 5'-CCTAGCCAGGGACGTTTTTC-3', 5'-TAGACAGACTGAGCGCCACC-3' for TGF β-2, all five 515, 310, 1080, 360, and 230 base pairs of the PCR products for each gene were obtained. The PCR reaction was carried out at different times and temperatures for each reaction period using the Perkin Elmer Cetus DNA Thermal Cycler (Model 2400, Foster City, CA, USA). These amplified PCR products were visible as a fluorescent band under UV rays, after agarose gel electrophoresis, at different time intervals and with ethidium bromide staining. Polaroid photographs were scanned using Epson GT-9500 (Seiko Corp, Nagano, Japan) and quantitated by densitometry (Image PC alpha 9, NIH, Bethesda, MD, USA) and the values were revised based on the GAPDH.
Protein extraction
Alcohol (100%) was added to the interface and the organic phase remained from the RNA separation; then the DNA portion was precipitated after the sample was centrifuged at 5,000 rpm for 5 min. Isopropanol was added to the remaining ethanol upper layer, which was centrifuged and dissolved in 0.3 M guanidine hydrochloride in 95% ethanol. Next, this layer was washed three times and a protein pellet was obtained. The extracted protein was dissolved in 1% SDS solution and preserved at -20℃. The Bradford method was used for the quantification of the protein.
Western blotting
To precipitate the DNA, the interface and the organic phase remaining after the RNA extraction were centrifuged at 5,000 rpm for 5 min at 4℃ after treatment with 100% alcohol. A residual phenol-ethanol upper layer was added with isopropanol, centrifuged and treated with 0.3 M guanidine hydrochloride dissolved in 95% ethanol. This layer was centrifuged at 10,000 rpm for 10 min at 4℃ after incubation at room temperature for 15 min, and this process was repeated three times. The sample was then washed to obtain a protein pellet. The extracted proteins were solubilized in 5×SDS loading buffer for 5 min at 95℃ and separated by electrophoresis on 10% SDS-polyacrylamide gels under reducing conditions. Subsequently, the proteins were transferred to nitrocellulose membranes (Amersham Life Science, Buckinghamshire, England). The nitrocellulose membranes were blocked in 5% skim milk with TBS-T (0.05% Tween 20 in 50 mM Tris, 150 mM NaCl, 0.05% NaN3, pH 7.4) at room temperature for 1 hr. The membranes were then incubated for 18 hr at 4℃ with the respective primary antibodies against clusterin (1:100 dilution), p38 (1:1,000 dilution), p53 (1;200 dilution), TGF β-2 (1:200 dilution), ERK 1/2 (1:1,000 dilution), and JNK-2 (1:500 dilution). Thereafter, the membranes were washed two times with TBS-T and incubated for 40 min with an anti-rabbit IgG (Amersham Life Science) at room temperature. After washing with TBS-T four times, the secondary antibody bound to the nitrocellulose was detected by incubation for 1 min with a detection reagent (Amersham Life Science) and then exposed to medical X-ray film (Agfa, Mortsel, Belgium) for 1 min. The film was developed by a FPM-3500 Fuji X-ray Film Processor (Fuji, Otawara, Japan). The developed X-rays were scanned using the Epson GT-9500 (Seiko Corp) and quantitated by densitometry (Image PC α 9).
RESULTS
On day 7, the average body weight was 13.1±1.4 g in the S group and 15.8±1.1 g in the control group. The heart weight was 0.08±0.01 g in the S group and 0.11±0.01 g in the control group. The heart weight/body weight ratio was 0.005±0.0009 in the S group and 0.007±0.0008 in the control group. The body weights, heart weights and heart weight/body weight ratio of the S group were significantly reduced compared to the control group (P<0.05).
Proliferating cell nuclear antigen (PCNA) and apoptosis
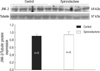
In PCNA immunostaining of the proliferating myocytes was lower in the myocardium of the neonatal rat heart in the S group compared to the control group. The PCNA-positive cells were 28.1±6.61 in the control group and 19.18±2.09 in the S group in each of the 25×25 µm fields (P<0.05, Fig. 1). Myocyte apoptosis was detected using the toatal TUNEL technique. The myocyte apoptosis was lower in the myocardium of neonatal rat heart in the S group compared to the control group. Apoptotic nuclei were present in 0.71±0.18 of the control group and in 0.50±0.08 of the S group for each 25×25 µm field (P<0.05, Fig. 2). Spironolactone treatment decreased proliferating myocytes by 32% (P<0.05) and reduced myocyte apoptosis by 30% (P<0.05). However, apoptotic nuclei accounted for only 2.5% of the relative fraction of cells undergoing proliferation in the present study. Finally, a net reduction of 32%, in cell proliferation, was observed in the S group compared to the control group.
Expression of p38
The semiquantitative RT-PCR showed that p38/GAPDH mRNA expression was 0.77±0.1 in the control group and 0.87±0.12 in the S group (P=0.18; Fig. 3A). Immunoblot analysis revealed that the p38/tubulin protein expression was significantly decreased in the S group (1.04±0.15) compared to the control group (1.33±0.19; P<0.05; Fig. 3B). Immnohistochemically, there was no difference between control and spironolactone group.
Expression of p53
The semiquantitative RT-PCR of p53/GAPDH mRNA expression was 1.02±0.11 in the control group and 1.13±0.08 in the S group (P=0.1; Fig. 4A). The immunoblot analysis revealed that the p53/tubulin protein expression was significantly decreased in the S group (0.71±0.11) compared to the control group (1.05±0.11; P<0.05; Fig. 4B).
Expression of clusterin
The semiquantitative RT-PCR showed that the clusterin/GAPDH mRNA expression was 0.90±0.22 in the control group and 0.93±0.17 in the S group (P=0.83; Fig. 5A). Immunoblot analysis revealed that the clusterin/tubulin protein expression was significantly decreased in the S group (0.91±0.14) compared to the control group (1.12±0.07; P<0.05; Fig. 5B). Immunohistochemically, there was no difference between control and spironolactone group.
Expression of TGF β
The semiquantitative RT-PCR showed that the TGF β-2/GA. PDH mRNA expression was 0.79±0.12 in the control group and 0.58±0.13 in the S group (P<0.05; Fig. 6). Immunoblot analysis revealed that the TGF β-2/tubulin protein expression was significantly decreased in the S group (0.83±0.15) compared to the control group (1.03±0.09; P<0.05: Fig. 6). Immunohistoche. mically, there was no difference between control and spironolactone group (Fig. 6). Immunoblot analysis showed that the TGF β-1/tubulin protein expression was 0.96±0.02 in the control group and 0.92±0.06 in the S group. There was no detectable difference between the two groups (Fig. 7).
Expression of ERK
The immunoblot analysis showed that the ERK-1/tubulin protein expression was 0.99±0.05 in the control group and 0.83±0.16 in the S group (Fig. 8A), and the ERK-2/tubulin protein expression was 0.85±0.07 in the control group and 0.89±0.07 in the S group (Fig. 8B). There was no detectable difference between the two groups.
Expression of JNK
Immunoblot analysis showed that the JNK-2/tubulin protein expression was 0.90±0.05 in the control group and 0.94±0.08 in the S group (Fig. 9). There was no detectable difference between the two groups.
DISCUSSION
The renin-angiotensin-aldosterone system (RAAS) plays an important role in regulating the cardiovascular system and is important in regulating the growth of the myocardium (13). Angiotensin II, a product of the RAAS, causes an increase in blood pressure through its vasoconstrictive effect. In addition, angiotensin II promotes cell growth via angiotensin II type I (AT1) receptors in cardiac myocytes, which cause inotropic and chronotropic changes and induce apoptosis in myocytes (14). A number of studies have demonstrated that alterations in the RAAS of developing rats affect cardiac growth and development (1, 15). A recent new wave of interest in aldosterone physiology has been generated by its role in cardiovascular homeostasis including blood-pressure homeostasis, myocardial fibrosis and remodeling of blood vessels (6). In addition, recent studies have shown that aldosterone induces cardiomyocyte apoptosis (6, 14). According to Mano et al. (8), there are plasma membrane receptors that are specific for aldosterone in myocytes. Activation of aldosterone receptors stimulates myocyte apoptosis through a cacineurin dependent mitochondrial death-signaling pathway. Chronic aldosterone receptor activation promotes vascular inflammation, vascular stiffness and perivascular collagen formation (16). The loss of myocytes by apoptosis in the dysfunctional heart results in reduced cardiac reserve capacity; this is one of the most important components of the pathogenesis of various cardiac diseases. Therefore, aldosterone excess has been shown to have a deleterious effect on the cardiovascular system (17).
However, there are few reports regarding the relationship between aldosterone and cardiac myocyte apoptosis and cell proliferation in the developing heart, especially in the neonate. In this study, both cell proliferation and apoptosis were decreased in the spironolactone treated group. Blocking of aldosterone reduced the number of proliferating myocytes by 32% and decreased myocyte apoptosis by 29%. The decrease in cell proliferation was counterbalanced by a reduction in myocyte apoptosis. However, the cells undergoing apoptosis accounted for only a minor fraction of the proliferating cells. Therefore, a net reduction of cell proliferation, in the cardiac myocytes, resulted from the blocking of aldosterone.
Although cardiomyocytes constitute only 30% of the ventricular cellular population in the mature rodent, they may occupy up to 85% of the total volume of the ventricular myocardium (18). Therefore, the net reduction of cell proliferation in myocytes, with aldosterone inhibition, may have contributed to the decrease in heart weight and heart weight to body weight ratio. The present in vivo animal study showed that aldosterone, the final product of RAAS, might play a role in cardiac growth in developing heart. The apoptotic and cell proliferation activity, in the cardiac myocytes, is mediated by several growth factors associated with apoptosis and cell proliferation. Among the MAPK signaling cascade, the p38 MAPK signaling pathway is activated in cardiac cells by hemodynamic stress or ischemia; it is thought to induce myocyte apoptosis, hypertrophy and fibroblast proliferation. In some animal models using transgenic mice, p38 activation resulted in interstitial fibrosis, cardiac hypertrophy and myocardial dysfunction (19). In the present study, p38 protein expression was significantly decreased by spironolactone; myocyte apoptosis was lower in the myocardium of the spironolactone treated group. These results suggest that p38 may be involved in the aldosterone-related intracellular signaling pathways of myocyte apoptosis in the developing heart.
p53 is a tumor suppressor protein; it has been reported to upregulate the cellular renin-angiotensin system, potentiating the synthesis and release of the octapeptide angiotensin II, which induces myocyte apoptosis via the activation of surface AT1 receptors (20). In addition, p53 is a transcriptional regulator of the proapoptotic gene product Bax and antiapoptotic gene product Bcl-2 (21), the activation of p53 may upregulate Bax and downregulate Bcl-2 in myocytes (22). The decrease in the Bcl-2: Bax ratio and upregulation of the cellular renin-angiotensin system, by the activation of p53, is thought to trigger myocyte apoptosis (23). The results of this study showed that p53 protein expression was significantly decreased. However, the p53 mRNA expression was not significantly different in the spironolactone treated group compared to the control group. These findings suggest that p53 is involved in the intracellular signaling pathways of myocyte apoptosis in the developing rat heart.
Clusterin is a disulfide-linked heterodimeric glycoprotein, first isolated from ram rete testis fluid in 1983, it has been reported to have a wide array of functions such as lipid transport, tissue remodeling, sperm maturation, and complement regulation (24). Recent studies have demonstrated that clusterin has both anti-apoptotic and anti-proliferative activity (25). The role of clusterin may vary, but it may be anti-proliferative or anti-apoptotic. If an anti-proliferative effect of clusterin dominates, this would render the cells susceptible to apoptosis. In this study, while there was no significant effect of spironolactone on myocardial expression of clusterin mRNA, clusterin protein expression was significantly decreased by treatment with spironolactone. These observations suggest that clusterin may participate in aldosterone-modulated intracellular signaling processes in myocyte apoptosis and proliferation.
The protein expressions of p38, p53, clusterin were found to be decreased significantly in the spironolactone group, however, their mRNA expression levels showed no significant difference compared with the control group. We suspected that mRNA expressions of p38, p53, culsterin were slightly increased -although there was no statistical significance- as a secondary response to the decreased cellular proliferation and decreased protein expressions.
Transforming growth factor-β (TGF-β) is a multifunctional growth factor molecule; it regulates cell proliferation and differentiation in many different cell types (26). Recent studies have demonstrated that TGF-β promotes cardiac myocyte differentiation from embryonic stem cells and upregulates cardiac transcription factors (27). The TGF-β family of peptides has three isoforms of TGF-β (TGF-β1, -β2, and -β3). Among these isoforms of TGF-β, TGF-β2 participates in cardiac myocyte differentiation. TGF-β2-knockout mice have severe cardiovascular malformations (28). In the present study, we found that the TGF-β2 protein and mRNA expression were significantly decreased in the spironolactone-treated group compared to the control group. These results suggest that TGF-β2 is involved in aldosterone-related intracellular signaling pathways of cardiac growth and myocyte proliferation.
It is known that ERK and JNK are important regulators of cardiac myocyte hypertrophic growth (29). ERK 1/2 activation is occurred to protect cardiomyocytes from apoptotic stimuli. However, there are no significant difference of ERK 1/2 expression between two groups in our experiment. This suggest that ERK singnaling pathway may not be associated with the action of spironolactone.
The activation of JNK signaling pathway is associated with cardiac hypertrophic response (30). However, there are no significant difference of JNK expression between two groups in our experiment. This suggest that JNK singnaling pathway may not be associated with the action of spironolactone.
In conclusion, the results of this study show an influential role of the aldosterone for the normal physiologic growth and development of the rat heart. The blocking of aldosterone in the developing heart causes cardiac growth impairment by decreasing cell proliferation and apoptosis of cardiac myocytes. In addition, the expression of p38, p53, clusterin, and TGF-β2 proteins is decreased. These findings suggest that aldosterone is important for normal cardiac growth, and that p38, p53, clusterin, and TGF-β2 may play an important role in the impairment of cardiac growth. In addition, aldosterone may post-transcriptionally modulate the expression of p38, p53, clusterin, and TGF-β2 during cardiac development. Further studies are needed to investigate the intracellular signaling mechanisms involved in a variety of metabolic pathways responsible for reduction of cell proliferation and apoptosis by aldosterone inhibition in the developing heart.




 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download