Abstract
We investigated the use of ulinastatin in association with the suppression of polymorphonuclear leukocyte elastase (PMNE), tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), and its effects on the prognosis of patients with traumatic hemorrhagic shock. Nineteen patients who visited the emergency department for traumatic hemorrhagic shock were enrolled. Eleven patients were randomly selected to receive a total of 300,000 IU of ulinastatin. Measurements of serum PMNE, TNF-α and IL-6 were taken before ulinastatin treatment at 24 hr, two days, three days, and seven days after admission. We compared the Systemic Inflammatory Response Syndrome scores, Multiple Organ Dysfunction Syndrome scores and Acute Physiology, age, Chronic Health Evaluation III scores of the control and ulinastatin groups. There were no significant differences in baseline values, laboratory data, treatment or mortality between the two groups. The serum PMNE levels in the ulinastatin group were lower than in the control group on the second hospitalized day. Serum TNF-α and IL-6 levels in the ulinastatin group decreased 24 hr after admission but had no significance. It is suggested that ulinastatin treatment could decrease the serum PMNE levels in trauma patients with hemorrhagic shock at 48 hr after treatment.
Shock resulting from hemorrhage induces life-threatening ischemic injuries in organs as well as tissues. This damage occurs during the resuscitation period, when neutrophils become active and inflammatory cytokines increase in both hemorrhagic shock and systemic inflammation (i.e. burn, acute pancreatitis, sepsis). These systemic metabolic changes can lead to acute respiratory distress syndrome and microischemia of the liver, and can impair functions of the kidney, heart and brain. Multiple organ failure is the leading cause of mortality after hemorrhagic shock (1-3).
Levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and polymorphonuclear leukocyte elastase (PMNE) begin to increase in early inflammation, stimulating various tissues and organs and leading to a systemic inflammatory response. Riedemann et al. (4) revealed protective effects of IL-6 blockade in sepsis.
Ulinastatin, a glycoprotein with a molecular weight of 67,000 daltons derived from human urine, has anti-inflammatory activity which may suppress PMNE, TNF-α, IL-6, IL-8 (5-8). In animal studies, the effects of ulinastatin on artificially induced hemorrhagic shock have been reported (8, 9), but studies about inflammatory cytokines or PMNE and clinical trials of hemorrhagic shock in humans have not been reported. The purpose of this study was to investigate the anti-inflammatory effects of ulinastatin on proinflammatory cytokines and PMNE in trauma patients suffering from hemorrhagic shock.
We conducted a randomized, controlled trial with a sample of 19 adult patients who were admitted to the emergency department at our hospital for trauma from June 2006 to October 2006. All enrolled patients suffered from traumatic hemorrhagic shock, arrived within six hours after an accident, and were 18 yr of age or older.
Patients with documented preexisting heart failure, chronic renal failure, liver cirrhosis or chronic obstructive pulmonary disease were excluded. Other exclusion criteria included cardiopulmonary resuscitation and severe brain injury, which was the main cause of death or morbidity. The enrolled patients were randomly divided into two groups, with patients admitted on odd numbered days assigned to the ulinastatin group and patients who were admitted on even numbered days assigned to the control group. Our study followed the guidelines of the Institutional Review Board of Wonju Christian Hospital, Yonsei University Wonju College of Medicine (No, CR 106072).
Ulinastatin was administrated to enrolled patients receiving blood transfusions and fluids immediately after diagnosis of hemorrhagic shock. Ulinastatin is commercially known as Ulistin® (Ulinastatin 100,000 IU/2 mL, Han Lim Pharm. Co. Ltd, Seoul, Korea). The ulinastatin group received 100,000 IU of ulinastatin with 100 mL of normal saline for duration of 30 min at a time, every eight hours, three times for a total of 300,000 IU of ulinastatin over 24 hr of treatment.
In the ulinastatin group, plasma concentrations of PMNE, IL-6 and TNF-α were measured before injection of ulinastatin, and at 24 hr, two, three, and seven days after injection. In the control group, plasma concentrations of PMNE, IL-6 and TNF-α were measured upon admission to the emergency room (ER), and at 24 hr, two, three, and seven days after admission. After centrifuging patient blood samples, the serum was stored in a freezer and then dissolved for Enzyme-Linked Immunosorbent Assay (ELISA; PhD™ System, Bio-Rad, Hercules, CA, USA).
Serum PMNE was measured in terms of the concentration of PMNE-α1-antitrypsin complex using a PMNE/α1-proteinase inhibitor complex ELISA kit (Calbiochem®, EMD Biosciences, Inc., Darmstadt, Germany). Serum IL-6 was measured using a Human IL-6 immunoassay kit (Quantikine®, R&D Systems, Inc., Minneapolis, MN, USA). Serum TNF-α was measured using a Human TNF-α/TNFSF1A immunoassay kit (Quantikine®, R&D Systems).
Upon admission and at 48 hr after admission to the ER, the Systemic Inflammatory Response Syndrome (SIRS) score, the Multiple Organ Dysfunction Syndrome (MODS) score, the Acute Physiology, Age, Chronic Health Evaluation (APACHE) III, transfusion amounts, cause of death and the duration of ICU admission of the control group and the ulinastatin group were compared (Fig. 1).
Data are reported as mean±standard deviation. Statistical analyses were performed with the Mann-Whitney U-test, Pearson's chi-square test for demographic data, and the Wilcoxon test for comparison between the SIRS score, the MODS score and the APACHE III score within the same group. Data were summarized and coded into a software program (SPSS v 13.0 for Windows, SPSS Inc., Chicago, IL, USA). P values of <0.05 was considered statistically significant.
The control group consisted of eight patients (five males, mean age 48.0±17.1 yr), and the ulinastatin group included eleven patients (eight males, mean age 48.7±11.1 yr). There were no significant differences in the two groups (P>0.05). Regarding the injury mechanism, eight were traffic accidents, three motorcycle accidents, five falls, one stabbing injury, one cultivator accident and one collision by rock. The Injury Severity Scores (ISS) for the two groups were 27.1±22.5 and 20.6±11.6, respectively (P=0.901). The Revised Trauma Scores (RTS) were 10.4±1.9 and 10.3±1.8, respectively (P=0.858). None of these differences were significant (Table 1).
White blood cell counts, neutrophil counts, hemoglobin, pH, base excess and lactate were not significantly different between the control and ulinastatin groups (P>0.05) (Table 2).
Serum TNF-α concentrations increased up to the third day of hospitalization in the control group and decreased thereafter such that by the seventh day, concentrations were lower than the initial concentrations obtained. Compared to the baseline level, the serum TNF-α levels of 1st, 2nd, 3rd, and 7th days were lower within the ulinastatin group. There were no significant differences between the mean concentrations of serum TNF-α in the control and ulinastatin groups (Fig. 2).
In the ulinastatin group, the serum concentration of IL-6 was 141.77±113.59 pg/mL at baseline and decreased to 52.82±29.68 pg/mL at 24 hr. The control group showed an increase in serum IL-6 from 78.31±52.95 pg/mL at baseline to 100.70±42.57 pg/mL at 24 hr. Although the IL-6 level of the ulinastatin group decreased more at HD1 than that of the control group, there was no significant difference (P>0.05) (Fig. 3).
The mean concentration of serum PMNE in the control group increased continuously and was 11.58±5.57 ng/mL on the second day of hospitalization. In the ulinastatin group, serum PMNE also increased showing a small difference and lower average. The mean plasma concentration of PMNE on the second hospital day in the ulinastatin group was 4.33±1.21 ng/mL, which was significantly lower than that of the control group (P=0.019).
The difference between the second hospitalized day and the day of admission was statistically significant between two groups (P=0.045) (Fig. 4)
Within the 24 hr after admission to ER, the total transfusion amounts of packed red blood cells, fresh frozen plasma, and platelet concentration were not different between the two groups (P>0.05). Within the control group, six of the eight patients underwent an operation, one had conservative treatment, and one patient died of hemorrhagic shock. In the ulinastatin group, six patients were treated conservatively and five underwent operations. Two patients died: one of hemorrhagic shock and the other of multiple organ dysfunction syndrome (Table 3). The treatment modalities in both groups were not significantly different (P=0.117).
In the ulinastatin group, the SIRS scores and APACHE III values decreased significantly over 48 hr (2.3±0.9 vs. 0.8±0.9, P=0.03; 42.7±28.6 vs. 24.9±23.8, P=0.02). MODS scores decreased after 48 hr of admission, but there was no difference between the treated and control groups (4.0±3.7 vs. 2.3±3.2, P=0.10). In the control group, SIRS and APACHE III scores decreased as well (2.8±1.0 vs. 0.6±0.8, P=0.03; 45.0±28.2 vs. 16.9±13.1, P=0.02). However, MODS scores remained stable (2.3±1.5 vs. 2.5±2.3, P=0.59) (Table 4).
The means of SIRS, MODS, and APACHE III scores were not significantly different between the two groups (Table 5).
One hundred years ago it was reported that human urine had the capacity to inhibit trypsin (10). In 1955, the protein responsible for the antitrypsin activity of urine, bikunin, was isolated (10). One main function of bikunin is to inhibit serine protease, especially elastase, and to suppress neutrophils, lymphocytes and macrophages increased by infection and inflammation (11). Now commonly known as ulinastatin, this urinary trypsin inhibitor inhibits cell apoptosis by free radicals and lipid peroxidation in renal ischemia-reperfusion injuries and has a suppressive effect against mitochondrial injury (8).
The effect of ulinastatin is dose-dependent (6, 12, 13). In this study, 100,000 IU, three times per day (total 300,000 IU) was administered as this is the recommended dosage for acute circulatory failure. This is comparable to the 50,000 IU/kg administered in canine experiments (9, 14), and 1,500,000 IU over a period of five days administered in clinical studies (7, 15). In Japan, ulinastatin 6,000 IU/kg is considered as the maximum safe dose (16). Although the possible side effects of ulinastatin include nausea, vomiting and hypersensitivity reactions, we did not observe any of these side effects in our sample, nor have they been observed in others. In most animal studies, ulinastatin was administered before the induction of hemorrhagic shock or septic shock and in clinical trials, before laparotomy or blood transfusion (5, 9, 14). In the present study, ulinastatin was prescribed only after diagnosis of traumatic hemorrhagic shock.
We examined levels of serum TNF-α, IL-6, PMNE as inflammatory mediators associated with hemorrhagic shock. Serum TNF-α is secreted by activated macrophages, stimulating other inflammatory cytokines and bringing inflammatory cells to tissues (17). IL-6 is secreted from the cells by an early inflammatory reaction. In a rat model, trauma-induced hemorrhagic shock led to increased plasma levels of the liver enzyme, alanine aminotransferase (ALT), a marker of liver injury, showing a significant correlation with IL-6 (18). Witthaut et al. (19) reported that serum IL-6 values in septic shock were 150 times higher than those of controls, and concluded therefore that IL-6 was the main cytokine of infection and inflammation. Neutrophils secrete PMNE when inflammation occurs, which can injure every tissue and organ (20, 21).
Levels of proteases such as elastase are typically increased in cases of inflammation and/or infection, and any substance that can inhibit this protease results in an anti-inflammatory effect (22). α1-protease inhibitor (α1-PI) and ulinastatin are intrinsic physiologic protease inhibitors that suppress PMNE activity. In inflammatory tissues, α1-PI loses its ability to function in acidic conditions, but ulinastatin can continue to inhibit PMNE (23). In addition, ulinastatin protects endothelial cells against neutrophil-mediated injury not only by inactivating the extracellular elastase secreted by neutrophils, but also by acting directly on neutrophils and suppressing the production and secretion of activated elastase (13).
Toth et al. (18) reported that ALT was suppressed by two thirds after injecting anti-IL-6 in resuscitated rats with hepatic injury from trauma-induced hemorrhagic shock. Vallejo et al. (24) found that treatment with a TNF-α receptor antagonist abrogated inflammatory mediators and left ventricular dysfunction before hemorrhagic shock or at the time of resuscitation.
Ulinastatin has demonstrated its effectiveness in animal models of experimentally induced septic and hemorrhagic shock (9, 14). In a septic shock canine model, ulinastatin improved blood pressure and lactic acid levels. Interestingly, although ulinastatin does not have anti-microbial activity, the ulinastatin-treated group was found to have a bacterial count that was significantly decreased and a higher survival rate (14). It is thought that ulinastatin might activate the reticuloendothelial system and phagocytosis (14). In hemorrhagic shock, the protective effects of ulinastatin might be associated with the up-regulation of Bcl-2, an inhibitor of the cell apoptosis (8).
Based on the literature, we hypothesized that ulinastatin would inhibit inflammatory cytokines such as TNF-α, IL-6 and PMNE, but in actuality, we found no differences among the averages of TNF-α, IL-6 and PMNE concentrations except for PMNE on the second day of hospitalization.
Aosasa et al. (6) reported that ulinastatin decreased the TNF-α production of lipopolysaccharide (LPS)-stimulated monocytes, but that decrease was not statistically significant. Serum TNF-α concentrations were low when ulinastatin was used before LPS stimulation and the serum concentration of TNF-α was inversely proportional to the amount of ulinastatin. In this study, after ulinastatin was injected in the ulinastatin group, the serum TNF-α levels were lower than initial serum TNF-α levels. Nonetheless, serum TNF-α concentrations in the control group increased until the third hospitalized day.
Serum IL-6 concentrations decreased by half after one day of ulinastatin injections in the ulinastatin group. Nishiyama et al. (5) proposed that ulinastatin might inhibit blood transfusion-induced increases of serum PMNE concentrations but not IL-6 after laparotomy. In their study, serum PMNE levels increased to a lesser extent than the control group by 50 percent. Tani et al. (15) used a total of 1,500,000 IU of ulinastatin for five days after laparotomy. In control and urinary trypsin inhibitor (UTI) groups, the rate of PMNE production per one leukocyte was not significantly different until the third day of postoperation but it was significantly lower in the UTI group than in the control group at fifth day. In another study, laparotomy was performed and ulinastatin was administered to the patients at the same time. In this instance, although serum PMNE levels did not decrease significantly, coagulation and fibrinolysis was significantly inhibited (16).
The reference range of serum PMNE levels was reported to be 20-180 µg/L (25) or 21-165 µg/L (5). We did not check the normal serum concentration of PMNE in our study, but the reference range was 0.15-3.0 ng/mL. The lowest value was 1.49 ng/mL and the highest value was 19.88 ng/mL. In clinical studies of the effects after laparotomy or blood transfusion, the peak values of serum PMNE were seen immediately after operations or blood transfusions. The serum concentrations of PMNE was significantly lower in the ulinastatin administration patients with gasterctomy and blood transfusion, but ulinastatin administration was not seen to decrease serum concentrations of PMNE (5). In another study, ulinastatin was not shown to decrease serum PMNE levels, but the activation of coagulation and fibrinolysis in abdominal surgery was inhibited (16). In this study, serum PMNE levels increased more than two-fold on the second hospitalized day within the control group. In addition, serum IL-6 values were highest upon admission to the ER and then gradually decreased in the ulinastatin group; however in the control group, serum IL-6 levels after one day of hospitalization were at their highest value and then subsequently decreased. In the rat experiment of hemorrhagic shock serum IL-6 concentrations increased until 24 hr after shock (18), as the serum IL-6 level at 24 hr was the highest in the control group in our study.
The laboratory data, SIRS, MODS and APACHE III scores, and mortality rates were not different between the two groups. This may be due to the small sample size. It may also be because the control group ultimately underwent the same medical procedures as the ulinastatin group, including blood transfusions, fluid therapy, and surgery. Therefore, our study should be replicated using a larger sample size and a longer period for clinical study. Furthermore, studies examining various dosages of ulinastatin for traumatic hemorrhagic shock patients are warranted.
It is suggested that ulinastatin treatment could decrease the serum PMNE levels in trauma patients with hemorrhagic shock at 48 hr after treatment. As mentioned previously, for the more positive results, a larger sample size, a longer period and other dosages of ulinastatin treatment studies are needed.
Figures and Tables
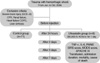 | Fig. 1The study design.
GCS, Glascow coma scale; CPR, cardiopulmonary resuscitation; COPD, chronic obstructive pulmonary disease; TNF, tumor necrosis factor; IL, interleukin; PMNE, polymorphonuclear leukocyte elastase; SIRS, systemic inflammatory response syndrome; MODS, multiple organ dysfunction syndrome; APACHE, acute physiology, age, chronic health evaluation.
|
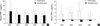 | Fig. 2(A) Effects of ulinastatin on serum TNF-α levels. (B) Changes in serum TNF-α levels after admission. HD0 means before injection of ulinastatin.
HD, hospitalized day.
|
 | Fig. 3(A) Effects of ulinastatin on serum IL-6 levels. (B) Changes in serum IL-6 levels after admission. HD0 means before infusion of ulinastatin.
HD, hospitalized day.
|
 | Fig. 4(A) Effects of ulinastatin on serum PMNE levels. (B) Changes in serum PMNE levels after admission. HD0 means before infusion of ulinastatin.
HD, hospitalized day.
|
Table 3
Transfusion, treatment modality and final results between the control group and the ulinastatin group

References
1. Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000. 26:Suppl 1. S64–S74.

2. Maitra SR, Gestring M, el-Maghrabi MR. Alternations in hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and glucose-6-phosphatase gene expression after hemorrhagic hypotension and resuscitation. Shock. 1997. 8:385–388.
3. Lee CC, Marill KA, Carter WA, Crupi RS. A current concept of trauma-induced multiorgan failure. Ann Emerg Med. 2001. 38:170–176.

4. Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced c5a receptor expression. J Immunol. 2003. 170:503–507.

5. Nishiyama T, Hanaoka K. Do the effects of a protease inhibitor, ulinastatin, on elastase release by blood transfusion depend on interleukin 6? Crit Care Med. 2001. 29:2106–2110.

6. Aosasa S, Ono S, Mochizuki H, Tsujimoto H, Ueno C, Matsumoto A. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor a production of monocytes. Shock. 2001. 15:101–105.
7. Sato Y, Ishikawa S, Otaki A, Takahashi T, Hasegawa Y, Suzuki M, Yamagishi T, Morishita Y. Induction of acute-phase reactive substances during open-heart surgery and efficacy of ulinastatin. Inhibiting cytokines and postoperative organ injury. Jpn J Thorac Cardiovasc Surg. 2000. 48:428–434.
8. Chen CC, Liu ZM, Wang HH, He W, Wang Y, Wu WD. Effect of ulinastatin on renal ischemia-reperfusion injury in rats. Acta Pharmacol Sin. 2004. 25:1334–1340.
9. Ohnishi H, Suzuki K, Niho T, Ito C, Yamaguchi K. Protective effects of urinary trypsin inhibitor in experimental shock. Jpn J Pharmacol. 1985. 39:137–144.

10. Fries E, Blom AM. Bikunin-not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000. 32:125–137.
11. Pugia MJ, Lott JA. Pathophysiology and diagnostic value of urinary trypsin inhibitors. Clin Chem Lab Med. 2005. 43:1–16.

12. Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003. 98:465–473.

13. Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Sekine I. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc Biol. 2001. 69:241–247.
14. Tani T, Aoki H, Yoshioka T, Lin KJ, Kodama M. Treatment of septic shock with a protease inhibitor in a canine model: a prospective, randomized, controlled trial. Crit Care Med. 1993. 21:925–930.
15. Tani T, Abe H, Endo Y, Hanasawa K, Kodama M. Effects of a urinary trypsin inhibitor on acute circulatory insufficiency after surgical operation. Am J Surg. 1998. 175:142–145.

16. Nishiyama T, Yokoyama T, Yamashita K. Effects of a protease inhibitor, ulinastatin, on coagulation and fibrinolysis in abdominal surgery. J Anesth. 2006. 20:179–182.

18. Toth B, Yokoyama Y, Schwacha MG, George RL, Rue LW 3rd, Bland KI, Chaudry IH. Insights into the role of interleukin-6 in the induction of hepatic injury after trauma-hemorrhagic shock. J Appl Physiol. 2004. 97:2184–2189.

19. Witthaut R, Busch C, Fraunberger P, Walli A, Seidel D, Pilz G, Stuttmann R, Speichermann N, Verner L, Werdan K. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis associated left ventricular dysfunction. Intensive Care Med. 2003. 29:1696–1702.
20. Duswald KH, Jochum M, Schramm W, Fritz H. Released granulocytic elastase: an indicator of pathobiochemical alterations in septicemia after abdominal surgery. Surgery. 1985. 98:892–899.
21. Pacholok SG, Davies P, Dorn C, Finke P, Hanlon WA, Mumford RA, Humes JL. Formation of polymorphonuclear leukocyte elastase: alpha 1 proteinase inhibitor complex and A alpha (1-21) fibrinopeptide in human blood stimulated with the calcium ionophore A23187. A model to characterize inhibitors of polymorphonuclear leukocyte elastase. Biochem Pharmacol. 1995. 49:1513–1520.
22. Mania-Pramanik J, Potdar SS, Vadigoppula A, Sawant S. Elastase: a predictive marker of inflammation and/or infection. J Clin Lab Anal. 2004. 18:153–158.

23. Ogawa M, Nishibe S, Mori T, Neumann S. Effect of human urinary trypsin inhibitor on granulocyte elastase activity. Res Commun Chem Pathol Pharmacol. 1987. 55:271–274.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download