Abstract
This is the first case of virus-associated encephalitis/encephalopathy in which the pathogen was Hantaan virus. A 53-yr-old man presented fever, renal failure and a hemorrhagic tendency and he was diagnosed with hemorrhagic fever with renal failure syndrome (HFRS). In the course of his illness, mild neurologic symptoms such as dizziness and confusion developed and magnetic resonance images revealed a reversible lesion in the splenium of the corpus callosum. This case suggests that HFRS patients with neurologic symptoms like dizziness and mental slowing should be considered to have structural brain lesions and to require brain imaging studies.
Reversible focal lesions in the splenium of the corpus callosum (SCC) have been found in patients with various conditions including seizures, antiepileptic drug toxicity or withdrawal, viral encephalitis, hypoglycemia, Wernicke's encephalopathy, Marchiafava-Bignami disease, sympathomimetic-induced kaleidoscopic visual illusion syndrome, hemolytic uremic syndrome, altitude brain injury, and acute axonal injury. Analysis of the current literature revealed that the most frequent cause of reversible focal lesions of the SCC is influenza virus-associated encephalitis/encephalopathy (IAEE). The abnormality has been reported in association with various viruses (e.g., Epstein-Barr virus, rotavirus, influenza virus) (1-3).
The brain lesions described in patients with IAEE include restricted diffusion involving the cerebral cortex and subcortical white matter in various locations, symmetric lesions in the brainstem, basal ganglia, thalamus, and cerebellar white matter with or without brain edema, and mild brain atrophy. Transient restricted diffusion of the SCC in patients with IAEE has also been described (4).
Here, we report an adult who presented with non-specific dizziness and lesions of the SCC on magnetic resonance imaging (MRI) who was diagnosed with hemorrhagic fever with renal failure syndrome (HFRS) with serologically proven Hantaan virus infection.
A previously healthy 53-yr-old man presented with a 6-7-day history of fever up to 40℃ and dizziness. He was admitted to a local hospital and underwent MRI. The initial diffusion-weighted images revealed high signal intensity in the corpus callosum with a low signal on the apparent diffusion coefficient (ADC) map (Fig. 1). The next day, he developed thrombocytopenia (40×103/µL) and was transferred to our emergency room for further evaluation.
He stated that he had been tying rice straw into sheaves several days earlier, but not using any drugs, like metronidazole, antiepileptic drug or alcohol, and had not been exposed to toxins or experienced weight loss.
On neurologic examination, he was slightly confused, and scored 24/30 on the mini mental status exam (MMSE). The cranial nerve, motor, and sensory examinations, deep tendon reflexes, and cerebellar function tests were normal.
His blood tests showed marked thrombocytopenia (21×103/µL) and azotemia (BUN/Cr 23 mg/dL/1.5 mg/dL). He was started on cefotaxime and clindamycin for a suspected infection and transfused with fresh frozen plasma, but his fever (>38.0℃) was sustained. Two days after admission, he was alert and his fever had subsided, but the oliguria, azotemia (BUN/Cr 49 mg/dL/3.0 mg/dL), and thrombocytopenia (6×103/µL) had worsened (Figs. 2, 3). Four days later, the thrombocytopenia had recovered, although the azotemia (BUN/Cr 82 mg/dL/7.1 mg/dL) had not improved. He was diagnosed with HFRS based on the detection of Hantaan virus antibody in his serum. His condition improved quickly after hemodialysis. Six days later, he was alert, and his electroencephalograph (EEG) was normal. His platelet count had stabilized (311×103/µL), and a lumbar puncture was performed. The cerebrospinal fluid (CSF) analysis revealed no cells, a nor mal protein level of 26.0 mg/L, a normal glucose level of 80 mg/dL, and no organisms on Gram staining. The MRI findings had resolved completely in follow-up studies conducted on day 14 (Fig. 4).
Hemorrhagic fever with renal failure syndrome occurs mainly in Europe and Asia and is characterized by a fever and renal failure associated with hemorrhagic manifestations. HFRS is caused by airborne contact with secretions from rodent hosts infected with viruses belonging to the genus Hantavirus in the family Bunyaviridae. The clinical features consist of a triad of fever, hemorrhage, and renal insufficiency (5). Other common symptoms during the initial phase of the illness include, myalgia, abdominal and back pain, and diarrhea. In most cases, mild neurological symptoms such as headache, vertigo, and nausea are common (6). The pathogenesis is largely unknown, but findings from several studies have suggested that immune mechanisms play an important role. After the infection, marked cytokine production, kallikrein-kinin activation, complement pathway activation, and increased levels of circulating immune complexes occur. These components play an important role during the febrile and hypotensive stages. Damage to the vascular endothelium, capillary dilatation, and leakage are clinically significant features of the disease.
The SCC has an irregular water component and low homogeneity. Myelin sheaths in the SCC have a relatively high water component, and the SCC is more susceptible to cytotoxic edema than are other brain areas (2). Reversible brain lesions have been attributed to the transient development of intramyelinic edema due to the separation of myelin layers, which is a possible mechanism for the transiently decreased diffusion of the SCC lesion (2, 11). Another possible explanation is the development of an inflammatory infiltrate. The influx of inflammatory cells and molecules, possibly combined with related cytotoxic edema, might have decreased the ADC (2, 12, 14).
The most common causes of reversible focal lesions of the SCC are viral encephalitis, antiepileptic drug toxicity/withdrawal, and hypoglycemic encephalopathy. Many other causes have been reported, including traumatic axonal injury. Influenza virus infections are a frequent cause of reversible splenium lesions (1). However, they are not specific to IAEE and have been reported secondary to various infectious agents, including rotavirus, measles, herpesvirus, Salmonella organisms, mumps, Varicella-zoster virus, adenovirus, O157 Escherichia coli-associated hemolytic-uremic syndrome, Legionnaires' disease, and unknown pathogens (4, 9, 14). The similar clinical and imaging features suggest a common mechanism induced by different pathological events leading to the same results (1). The most likely causes of these transient lesions of the SCC have been explained as a rapidly resolving intramyelinic infiltrate or the influx of inflammatory cells and macromolecules, combined with related cytotoxic edema, which is very similar to the pathogenesis of HFRS (2, 7, 9, 10, 13).
Those cases were also followed by complete clinical and MRI recovery. This argues for a non-specific cause related to vasogenic edema, probably secondary to the inflammatory response (2, 7)
Our patient was diagnosed with HFRS, and his MRI showed SCC lesions. We report a reversible SCC lesion associated with HFRS. To our knowledge, this is the first case of virus-associated encephalitis/encephalopathy in which the pathogen was a Hantaan virus. Although it is not certain whether the neurologic symptoms in this patient might have been caused by fever, or SCC lesions, or both, we suggest that HFRS patients with neurologic symptoms like dizziness and mental slowing should be considered to have structural brain lesions and to require brain imaging studies.
Figures and Tables
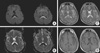 | Fig. 1Initial MRI. Diffusion-weighted images (A) show a high signal intensity in the splenium of the corpus callosum (SCC). ADC map images (B) at the same level show a low ADC, reflecting restricted diffusion. T2-weighted images (C) reveal increased signal intensity in the SCC lesion with slightly decreased signal intensity on T1-weigted images (D). |
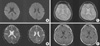 | Fig. 4Follow-up MRI obtained 14 days later. The images correspond to those in Fig. 1 and show complete resolution of the SCC lesion with no residual changes. |
References
1. Gallucci M, Limbucci N, Paonessa A, Caranci F. Reversible focal splenial lesions. Neuroradiolog. 2007. 49:541–544.

2. Cho JS, Ha SW, Han YS, Park SE, Hong KM, Han JH, Cho EK, Kim DE, Kim JG. Mild encephalopathy with reversible lesion in the splenium of the corpus callosum and bilateral frontal white matter. J Clin Neurol. 2007. 3:53–56.

3. Tsuji M, Yoshida T, Miyakoshi C, Haruta T. Is a reversible splenial lesion a sign of encephalopathy? Pediatr Neurol. 2009. 41:143–145.

4. Fasano A, Natoli GF, Cianfoni A, Ferraro D, Loria G, Bentivoglio AR, Servidei S. Acute necrotizing encephalopathy: a relapsing case in a European adult. J Neurol Neurosurg Psychiatry. 2008. 79:227–228.

5. Choi HS, Lee YS, Hwang JC, Lim JH, Kim KS, Yoon Y. Renal artery embolization of perirenal hematoma in hemorrhagic fever with renal syndrome: a case report. Korean J Radiol. 2007. 8:348–350.

6. Cerar D, Avšič-Županc T, Jereb M, Strle F. Case report: severe neurological manifestation of Dobrava hantavirus infection. J Med Virol. 2007. 79:1841–1843.

7. Takanashi J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev. 2009. 31:521–528.

8. Fasano A, Natoli GF, Cianfoni A, Ferraro D, Loria G, Bentivoglio AR, Servidei S. Acute necrotizing encephalopathy: a relapsing case in a European adult. J Neurol Neurosurg Psychiatry. 2008. 79:227–228.

9. Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand Suppl. 2007. 186:45–56.

10. Sazgar M, Robinson JL, Chan AK, Sinclair DB. Influenza B acute necrotizing encephalopathy: a case report and literature review. Pediatr Neurol. 2003. 28:396–399.

11. Kohler CG, Ances BM, Coleman AR, Ragland JD, Lazarev M, Gur RC. Marchiafava-Bignami disease: literature review and case report. Neuropsychiatry Neuropsychol Behav Neurol. 2000. 13:67–76.
12. Kuo CJ, Chen LK, Peng HL, Wu CC. Marchiafava-Bignami disease: CT, MRI and 99mTc HMPAO-SPECT Findings. Chin J Radiol. 2004. 29:87–91.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download