Abstract
Erysipelothrix rhusiopathiae is known as a pathogen of occupational diseases or a zoonosis. We report a case of E. rhusiopathiae peritonitis in a 50-yr-old male undergoing continuous ambulatory peritoneal dialysis (CAPD). He was suffered from mild abdominal pain with a distinctive erysipeloid skin lesion. E. rhusiopathiae was considered to be introduced through a lacerated wound on his hand when he was exposed to contaminated materials. He was treated successfully with a first generation cephalosporin. To our knowledge, CAPD peritonitis due to E. rhusiopathiae is very rare, and this is a report of the first case in Asia.
Peritonitis is one of the most important complications in patients undergoing peritoneal dialysis (PD). Peritonitis is the main cause of hospitalization in PD patients, and is also associated with catheter loss, adhesions, change to hemodialysis, and considerable morbidity (1). Rapid and correct identification of the causative pathogen and antibiotic susceptibilities is critical to manage the patient undergoing continuous ambulatory peritoneal dialysis (CAPD) with peritonitis properly. The initial treatment of the CAPD patient with peritonitis is empirical, so it is difficult to treat the CAPD peritonitis that is caused by a rare organism, especially if the pathogen is resistant to the initial empirical antibiotics. Erysipelothrix rhusiopathiae is a gram-positive, rod-shaped microorganism, inherently resistant to vancomycin. Infection in humans is associated with occupations, and is caused by direct skin exposure to animal reservoirs or contaminated materials. The clinical manifestations vary from mild cutaneous infections to severe endocarditis or sepsis (2). We present the first case in Asia about E. rhusiopathiae CAPD peritonitis, with a review of two other cases reported in Europe and North America.
A 50-yr-old retired salesman living in an urban area with his lap dog visited our hospital because of abdominal pain and erythematous skin lesions. He had type 2 diabetes mellitus for 15 yr, which was complicated by end stage renal failure. PD had begun 5 yr earlier.
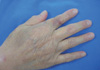
Four days before he visited the hospital, he sustained a small lacerated wound on his right index finger while trimming the nail, but did not seek care. The next day, he had mild abdominal pain, loose stools, and several bullous skin lesions that progressed from the right hand and forearm to the back area. He claimed that the exchanged CAPD fluid was clear. He thought the symptoms might be due to food poisoning because he ate raw fish the day before the symptoms developed. When he sought evaluation at our hospital, the vital signs were stable and he was afebrile. The initial bullous skin lesions that he described had almost resolved and were replaced with erythematous, violaceous macular lesions (Fig. 1). There was a diffuse abdominal tenderness, but without signs of peritoneal irritation. The CAPD fluid was slightly turbid. Both first and third generation cephalosporins were administered intraperitoneally according to the hospital recommendation for CAPD peritonitis. The initial cell count of the peritosol showed a white blood cell (WBC) of 1,600/µL (polycytes, 70%; lymphocytes, 12%; and others, 18%) with no red blood cells. Gram positive rods were demonstrated on gram stain. At the next visit on day 3, the abdominal pain was markedly improved. As a causative pathogen, E. rhusiopathiae was identified by a GNI-plus card with a Vitec II compact system (BioMeriux, Marcy-I'Etoile, France). The colonies on the blood agar plate were small and transparent, showing negativity for catalase, oxidase, and methyl red. On slants of triple sugar iron (TSI) agar, hydrogen sulfide was detected as a black precipitate. E. rhusiopathiae showed antibiotic susceptibility to cephalosporins, penicillin, erythomycin, and clindamycin, but resistance to vancomycin. The repeat examination of the CAPD fluid showed a WBC count of 42/µL (polycytes, 4%; lymphocytes, 17%; and others, 21%). He was treated successfully with intraperitoneal cefazolin, a first generation cephalosporin, for 2 weeks.
E. rhusiopathiae was first isolated from mice in 1878 and identified as a human pathogen in 1909 from a patient with localized cutaneous lesions. Human infection is considered to be a zoonosis, and is related to direct skin exposure to animal reservoirs, such as domestic swine, dogs, cats, fish, shellfish, sheep, and fowl. Animal reservoirs can contaminate the soil enough to cause infection for several months (3). The clinical manifestations vary according to severity and location. The most common manifestation is a localized skin infection (erysipeloid) and it should be distinguished from erysipelas, a superficial cellulitis caused exclusively by streptococci (2). Infective endocarditis or sepsis can develop in severe cases. According to the literature regarding in vitro antimicrobial susceptibilities, penicillin and imipenem are known as the most active agents, but vancomycin, teicoplanin, and gentamicin show poor or absent activity for E. rhusiopathiae (3).
There have been only two reported cases of CAPD peritonitis caused by E. rhusiopathiae. The first case was reported in a rancher who had a minor wound on his hand, through which E. rhusiopathiae may have been innoculated while touching a barbed wire fence enclosing animals (4). He had abdominal pain and an erythematous skin lesion. He was treated successfully with penicillin following the isolation of E. rhusiopathiae from the CAPD fluid. The second case was a 49-yr-old bricklayer, whose infection was considered to be through his excoriated hands when exposed to unknown contaminated materials (5). In contrast to the first case, the Tenchkoff PD catheter was removed because E. rhusiopathiae was resistant to the initial therapy with vancomycin. Recovery was achieved with the change of vancomycin to ciprofloxacin, to which E. rhusiopathiae was susceptible. Vancomycin has been used as initial empiric therapy depending on the experience of each center, but identification and determination of the antibiotic sensitivity of the isolated pathogen should not be overlooked, considering the pathogens which are inherently vancomycin-resistant, as in E. rhusiopathiae (6). In our case, there were two possible routes of entry. One was through the gastrointestinal tract with transient bacteremia, which is associated with ingestion of a raw fish. However, the probability was very low, because on inquiry the fish that he ate was a Pampus argenteus, which has not been reported as a reservoir of E. rhusiopathiae. The other possible route was through the small wound involving his right hand. The skin lesion started at this site with progression to the forearm and back, and abdominal pain developed simultaneously. Because he lived with a lap dog, it could be postulated that the skin wound was contaminated. Talan et al. (7) reported that E. rhusiopathiae was isolated in the bacteriologic analysis of the infected wounds caused by dog and cat bites. Shimazaki et al. (8) reported a 5% prevalence of E. rhusiopathiae in dogs with positive antibody titers against E. rhusiopathiae. If the infection source was related to the pet, more strict hygiene should be emphasized in dialysis patients, considering that more and more people live with pets, and the likelihood of contact animals or contaminated materials is on the rise.
This case emphasizes the importance of initial therapy and antibiotic susceptibilities in CAPD peritonitis. One should not dismiss the importance of identifying the causative pathogen and searching for the source of infection considering all of the possibilities, including unusual situations, such as zoonosis or environmental contamination.
Figures and Tables
References
1. Saklayen MG. CAPD peritonitis. Incidence, pathogens, diagnosis, and management. Med Clin North Am. 1990. 74:997–1010.

2. Brooke CJ, Riley TV. Erysipelothrix rhusiopathiae: bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J Med Microbiol. 1999. 48:789–799.

3. Venditti M, Gelfusa V, Tarasi A, Brandimarte C, Serra P. Antimicrobial susceptibilities of Erysipelothrix rhusiopathiae. Antimicrob Agents Chemother. 1990. 34:2038–2040.

4. Carlini ME, Clarridge JE, Rodriguez-Barradas MC. Erysipelothrix rhusiopathiae peritonitis in a patient on continuous ambulatory peritoneal dialysis. Infect Dis Clin Pract. 1998. 7:419–421.

5. Hardman SC, Carr SJ, Swann RA. Peritoneal dialysis-related peritonitis with bacteraemia due to Erysipelothrix rhusiopathiae. Nephrol Dial Transplant. 2004. 19:1340–1341.

6. Fidalgo SG, Longbottom CJ, Rjley TV. Susceptibility of Erysipelothrix rhusiopathiae to antimicrobial agents and home disinfectants. Pathology. 2002. 34:462–465.

7. Talan DA, Citron DM, Abrahamian FM, Moran GJ, Goldstein EJ. Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999. 340:85–92.
8. Shimazaki Y, Gamoh K, Imada Y, Makie H, Kanzaki M, Takahashi T. Detection of antibodies to Erysipelothrix in stray dogs in Japan. Acta Vet Scand. 2005. 46:159–161.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download