Abstract
Minocycline is a semisynthetic tetracycline derivative that is often used in the treatment of acne vulgaris. To date, there has been only one case report of anaphylaxis to minocycline. We report here a case of anaphylaxis to oral minocycline. A 56-yr-old woman visited our hospital after three episodes of recurrent anaphylaxis. We performed an oral challenge test, the standard method for diagnosing drug allergies, with minocycline, one of the drugs she had taken previously. She developed urticaria, angioedema, nausea, vomiting, hypotension, and dyspnea within 4 min and was treated with intramuscular epinephrine, intravenous antihistamine and systemic corticosteroid. However, she presented similar symptoms at 50 min and at 110 min. In prescribing oral minocycline, physicians should consider the possibility of serious adverse reactions, such as anaphylaxis.
Allergic reactions to antibiotics are associated with increased rates of morbidity and mortality, as well as increased medical costs (1-3). Anaphylaxis is a life-threatening reaction to drugs. Although many cases of anaphylaxis to penicillin and cephalosporin have been reported, anaphylaxis to tetracycline (4) is less common. Tetracycline is a broad-spectrum polyketide antibiotic produced by the Streptomyces genus of Actinobacteria, which has been indicated in the treatment of many types of bacterial infections. Although the use of tetracycline has decreased, minocycline, a semisynthetic, second-generation tetracycline derivative, is still used worldwide in the treatment of acne.
Although minocycline is relatively safe, it has been reported to have adverse effects in 13.6% of treated patients (5). Common adverse effects of minocycline include gastrointestinal disturbances (nausea and vomiting), vestibular disturbance (dizziness), and cutaneous symptoms (hyperpigmentation of skin and mucous membranes, pruritis, urticaria, and photosensitive rash). Serious but rare adverse effects of minocycline include pneumonitis, hepatitis, pancreatitis, nephritis, polyarthritis, serum sickness-like reaction, drug-induced lupus erythematosus-like eruption, and hypersensitivity syndrome (6, 7).
To our knowledge, only one case of anaphylaxis to oral minocycline has been reported to date (8). Here we report a case of anaphylaxis to oral minocycline, confirmed by an oral challenge test.
A 56-yr-old woman visited our hospital after experiencing three episodes of recurrent anaphylaxis during the previous year. In every episode, she presented with urticaria, angioedema, dyspnea, and hypotension and visited the emergency department. In the first episode, she had generalized skin rash with an itching sensation, periorbital and perioral swelling, dyspnea, and dizziness. Her blood pressure decreased to 73/46 mmHg and she was diagnosed with and treated for anaphylaxis. At that time, the precipitating cause was not evaluated. During the second and third episodes, however, she noticed that her symptoms developed within a few minutes after taking bepotastine besilate, methylprednisolone, and minocycline for acne.
Her initial laboratory tests at the first visit to our hospital revealed no apparent abnormalities except for mild leukocytosis (16,700/µL). Her total serum IgE concentration was 223 KU/L. Chest radiograph and electrocardiography were normal. Skin prick tests with inhalants and food allergens all showed negative results.
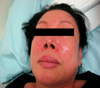
We performed an oral minocycline challenge test to confirm the causative drug of her recurrent anaphylaxis. The patient took a half capsule of Minocin [50 mg]® (SK Chemical Life Science, Seoul, Korea). Within 4 min, she began to feel an itching and burning sensation in her face and forearms, followed by the development of generalized wheal. She also presented with periorbital and lip swelling (Fig. 1). In 10 min she developed throat discomfort, dyspnea and wheezing. She was distressed because of nausea, vomiting and dizziness. On physical examination, we heard a wheezing sound in her whole lung field and an abrupt fall in blood pressure, to 70/50 mmHg. At that time, her pulse rate was 55 beats/min, her respiratory rate was 24/min, and her SpO2 was 93%. She was administered two 0.3 cc doses of epinephrine intramuscularly 5-min apart, along with intravenous chlorpheniramine (H1-antagonist) 4 mg, famotidine (H2-antagonist) 20 mg and hydrocortisone 250 mg. The patient also inhaled an albuterol nebulizer (short-acting B2-agonist). Ten minutes later, her blood pressure had increased to 105/70 mmHg. Thirty minutes later, however, she again developed hypotension (80/40 mmHg), for which she was treated with additional intramuscular epinephrine. After 1 hr, she again developed hypotension (80/40 mmHg) (Fig. 2), for which she was managed with the same regimen. On the following day, the patient had fully recovered without apparent complications and discharged. She was educated about avoidance of tetracycline and given a 'drug-alert card'. Since stopping minocycline, she has experienced no additional episodes of anaphylaxis.
Anaphylaxis is a rare, but serious, form of drug hypersensitivity. It is a systemic reaction mediated by vasoactive amines released from mast cells and basophils sensitized by immunoglobulin E (IgE). Immediate hypersensitivity reactions commonly involve at least two of the following major organ systems: cutaneous (generalized hives, pruritus, swollen lips-tongue-uvula), cardiovascular (hypotension), respiratory (dyspnea due to laryngeal edema, bronchospasm, stridor), and gastrointestinal (vomiting, diarrhea, abdominal cramps) (9). Individuals differ in the time of appearance of symptoms and signs, but the hallmark of an anaphylactic reaction is the onset of some manifestations within seconds to minutes after introduction of the antigen.
Several reports have described anaphylactoid reactions to tetracycline (4), doxycycline (10), and oral minocycline (8). In the latter report, a 27-yr-old woman diagnosed with salpingitis and treated with 100-mg oral minocycline developed generalized wheal and erythema, dyspnea, and hypotension within 30 min after taking minocycline. The symptoms cleared in about 4 hr, and a scratch test performed on her forearm with minocycline resulted in a positive reaction. In that case, however, an oral challenge test was not performed.
The pathogenesis of minocycline-induced hypersensitivity is unknown. These types of hypersensitivity reactions are thought to be caused by a reactive metabolite of a drug, which can bind to tissue macromolecules, causing cell damage, or can act as a hapten, eliciting an immune response. Although it is not known whether minocycline produces a reactive metabolite, it may generate an iminoquinone derivative. Neither tetracycline nor doxycycline contains the amino acid side chain that has the potential to form a reactive intermediate, and therefore the hypersensitivity associated with minocycline may be specific to this antibiotic. Further evaluation of the pathogenesis associated with minocycline is required (11).
To our knowledge, this is the first case report to describe anaphylaxis to oral minocycline in Korea. The immediate and typical anaphylactic symptoms and signs, the dramatic triphasic reactions after oral challenge, and the previous history of anaphylaxis after exposure to minocycline, indicate that anaphylaxis in this patient was mediated by type I hypersensitivity reactions. To our knowledge, this report is also the first to utilize an oral challenge test, the standard method for the diagnosis of drug allergies, to confirm that minocycline was the culprit drug.
Since minocycline remains a useful drug for the treatment of acne vulgaris, physicians should keep in mind that minocycline can induce anaphylaxis. In addition, this drug should be considered a causative agent in patients developing allergic reactions.
Figures and Tables
References
1. Adkinson NF Jr, Essayan D, Gruchalla R, Haggerty H, Kawabata T, Sandler JD, Updyke L, Shear NH, Wierda D. Task force report: future research needs for the prevention and management of immune-mediated drug hypersensitivity reactions. J Allergy Clin Immunol. 2002. 109:S461–S478.

2. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998. 279:1200–1205.

3. McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995. 273:214–219.

4. Barnett CF Jr. Anaphylactoid reaction to orally administered tetracycline. South Med J. 1967. 60:963.

5. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996. 134:693–695.

6. Clayton BD, Hitchcock MG, Williford PM. Minocycline hypersensitivity with respiratory failure. Arch Dermatol. 1999. 135:139–140.

7. Colvin JH, Sheth AP. Minocycline hypersensitivity syndrome with hypotension mimicking septic shock. Pediatr Dermatol. 2001. 18:295–298.

8. Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996. 76:164.
9. Chaudhuri K, Gonzales J, Jesurun CA, Ambat MT, Mandal-Chaudhuri S. Anaphylactic shock in pregnancy: a case study and review of the literature. Int J Obstet Anesth. 2008. 17:350–357.

10. Raeder JC. Anaphylactoid reaction caused by intravenous doxycycline during general anesthesia and beta-blockade treatment. Drug Intell Clin Pharm. 1984. 18:481–482.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download