Abstract
Body weight is positively associated with bone mineral density but the relationship between obesity and bone mineral density is unclear. Leptin and adiponectin are potential independent contributors to bone mineral density. We assessed the correlations of body composition, leptin and adiponectin with bone mineral density, and whether leptin, adiponectin and body composition determine bone mineral density independently in prepubertal girls. Forty-eight prepubertal girls were classified into obese and control groups by body mass index. Serum leptin and adiponectin levels were determined by enzyme immunoassay. Bone mineral density was measured using dual energy radiography absorptiometry and body composition was measured using bioelectrical impedance analysis. Lean and fat mass, and leptin were positively correlated with bone mineral density. Lean mass was a positive independent predictor of femoral and L-spine bone mineral density. Serum leptin was a postivie independent predictor of femoral bone mineral density. Fat mass was a negative independent predictor of femoral bone mineral density. In prepubertal girls, lean mass has a favorable effect on bone mineral density. Fat mass seems not to protect the bone structure against osteoporosis, despite increased mechanical loading. Serum leptin may play a biological role in regulating bone metabolism.
Childhood obesity has become a major public health concern. Obesity is a known risk factor for cardiovascular disease, diabetes, hypertension, and cancer (1, 2). However, the relationship between obesity and bone mineral density (BMD) is unclear (3).
Childhood and adolescence are crucial periods for maximum bone mass acquisition, which is associated with genetic potential, nutritional factors, physical activity, and body composition (4). Osteoporosis-related fracture risk is highly dependent on BMD (5). Body weight is positively associated with BMD (6) and negatively associated with fracture incidence (7). However, it remains controversial whether it is lean mass or adipose tissue that mediates the bone stimulatory effect exerted by weight (8, 9). Recent reports indicated that in young female populations, lean mass is a positive predictor of BMD, whereas adipose tissue is a weaker positive (10) or negative predictor of BMD (11-13). Adipose tissue secretes a variety of proteins called adipokines into circulation which play important roles in the modulation of biological functions. The relationship between adipose tissue and BMD is credited not only to stress from mechanical loading but also to the metabolic effect of adipokines (14). Leptin and adiponectin are potential contributors to BMD. Leptin is a satiety regulating hormone and has a central role not only in regulating energy expenditure, but also in bone metabolism (3), and increases the proliferation and differentiation of osteoblasts in adults (15). Adiponectin increases insulin sensitivity and may improve lipid profiles (16). It has been suggested that high adiponectin levels may cause increased osteoclastic activity and low BMD (17). Adiponectin, however, has also been reported to increase osteoblastic activity and decrease osteoclastic activity in animals (18).
The relationship between body composition, especially adipose tissue, and BMD in obese children is clinically important because any therapeutic interventions for obesity that modify body composition may affect BMD and therefore the risk of osteoporosis in later life. Few studies have attempted to investigate the effect of body composition and adipokines on BMD in children. Accordingly, we investigated the relationship between body composition, leptin, adiponectin, and BMD, and whether adipokines and body composition determine BMD independently in prepubertal girls.
This study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB number AS0768). Written informed consent was obtained from both subjects and parents. The children who participated in this study were recruited from prepubertal girls who visited the outpatient Pediatric Obesity Clinic of Department of Pediatrics, Korea University Ansan Hospital, Ansan, Korea. Pubertal stage was determined by a single pediatric endocrinologist according to the Tanner scale, and subjects who showed pubertal development were excluded. None of the participants in this study had a medical history of cardiovascular disease, diabetes, hypertension, and other endocrine disorders.
Height was measured to the nearest 0.1 cm using a rigid stadiometer. Weight was measured to the nearest 0.1 kg using a calibrated balance scale. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters. Obese subjects were defined as having a BMI greater than or equal to the 95th percentile for their age and sex according to 2007 Korean national growth chart. Control subjects were defined as having a BMI greater than or equal to the 25th percentile but less than the 75th percentile. Body composition measurements were performed by bioelectrical impedance analysis, using an InBody 4.0 body composition analyzer (Biospace, Seoul, Korea). Fat and lean mass were expressed in kilograms.
BMD was measured by a single technician using dual energy radiography absorptiometry (DXA), using Expert version 1.90 (Lunar Corp., Madison, WI, USA). BMD of the L2-L4 lumbar spine and femoral neck was evaluated and expressed in grams/centimeter2.
Blood sampling was performed after an overnight fast. Serum parathyroid hormone (PTH), osteocalcin, insulin, glucose, total cholesterol, triglyceride, LDL-cholesterol, HDL-cholesterol, leptin, and adiponectin were measured. Leptin and adiponectin levels were measured with enzyme immunoassay kits (ALPCO Diagnostics, Salem, NH, USA and AdipoGen, Inc., Seoul, Korea, respectively). Insulin resistance was estimated using a homeostatic model assessment of insulin resistance (HOMA-IR), with the following calculations: HOMA-IR=(insulin [mU/L]×glucose [mM/L])/22.5.
Data are expressed as mean ± standard deviation. Anthropometric characteristics, metabolic and biochemical parameters, body composition and BMD between obese and control groups were compared using an independent t-test. Pearson's correlation coefficients were calculated to evaluate the relationship between BMD and metabolic parameters and body composition. Multiple linear regression analysis was performed to investigate whether BMI, serum fasting insulin, leptin, adiponectin and body composition determine BMD independently. SPSS 16.0 software was used for statistical analysis. P values <0.05 were considered statistically significant.
Among 48 prepubertal girls who participated in this study, twenty-nine girls were classified into obese group and 19 girls were placed into control group. Anthropometric characteristics, metabolic and biochemical parameters, body composition and femoral and L-spine BMD of obese and control groups are shown in Table 1. Obese group had significantly higher weight, BMI, leptin, fat mass and femoral and L-spine BMD compared to control group. There were no significant differences in age, height, PTH, osteocalcin, lipid profiles, fasting glucose, insulin, HOMA-IR, adiponectin and lean mass between obese and control groups.
The results of Pearson's correlation of age, height, weight, BMI, fat and lean mass, serum leptin, adiponectin and insulin levels, with femoral and L-spine BMD are shown in Table 2. Body weight, BMI, fat and lean mass, and leptin level were positively correlated with femoral and L-spine BMD. Fasting insulin had positive correlations with femoral BMD but not with L-spine BMD. Height had positive correlations with L-spine BMD but not with femoral BMD. Age and serum adiponectin level were not correlated with femoral and L-spine BMD.
The results of multiple linear regression analysis to investigate whether BMI, leptin, adiponectin, fasting insulin and body composition determine BMD independently are shown in Table 3. Lean mass was found to be a positive independent predictor of femoral and L-spine BMD. Serum leptin was found to be a positive independent predictor of femoral BMD, but not of L-spine BMD. Fat mass was found to be a negative independent predictor of femoral BMD, but not of L-spine BMD. BMI, serum adiponectin and fasting insulin levels were not independent predictors of BMD.
The results of this study agree with previous studies that documented a positive relationship between body weight and BMD (6). The BMD of obese children is influenced by body weight as excessive weight produces a mechanical force on the bones, thereby stimulating osteogenesis (19).
Obesity is characterized by increased body weight with excess body fat and a relative increase of lean mass. It has been debated which of lean mass or fat mass has more influence on bone stimulatory effect (8, 20). Previous studies indicated that regardless of age or gender, lean mass has a strong positive influence on BMD (10). However, the results of previous studies on the relation between fat mass and BMD were conflicting. Adipose tissue can be a weaker positive predictor (10) or stronger predictor (6) than lean mass, or even a negative predictor of BMD (13).
Our results indicate that despite its positive correlation with femoral L-spine BMD, fat mass is a negative independent predictor of femoral BMD. This finding is consistent with previous reports suggesting that bone strength is primarily determined by dynamic loads from muscle force, not static loads such as fat mass (21). The mechanism for the negative effect of fat mass on bone mass, as observed in this study, is unknown.
Adipose tissue is no longer viewed as a metabolically passive fuel depot for energy substrate. Rather, it is a metabolically active tissue, secreting a variety of adipokines that modulate biological functions. It is suggested that some adipokines participate in bone metabolism. Leptin and adiponectin are potential contributors to BMD.
Leptin has been proposed to be a mediator of adipose tissue hormonal effect on bone mass (3). The role of leptin in bone metabolism is not fully understood, but in animal studies, leptin deficient mice have demonstrated a 'high bone mass phenotype' (22). Some human studies have failed to show any association between serum leptin levels and BMD (3, 23), whereas others have reported a positive association between leptin and BMD (24). In a few recent studies, leptin was negatively correlated with BMD (25). We found that serum leptin levels were positively correlated with both femoral and L-spine BMD, and that leptin was an independent positive predictor of femoral BMD in prepubertal girls.
Adiponectin acts directly on bone to induce human osteoblast proliferation and differentiation, and to increase osteoclast formation indirectly (17, 26). A previous study showed that adiponectin exerted a negative independent effect on BMD (27). Some studies also reported that there was no independent relationship between adiponectin and BMD (28, 29). We did not find any correlations between adiponectin and BMD.
Another link between obesity and BMD is insulin. Insulin reduces hepatic synthesis of sex hormone carriers, and leads to an increase in free form sex hormones, which stimulates the activity of osteoblasts (30). We found a weak positive correlation between fasting insulin and femoral BMD, but no significant correlation between fasting insulin and L-spine BMD.
We limited our study sample to prepubertal girls to control for other factors that affect BMD. In puberty, growth hormones and sex steroids actively participate in the development of bone structure as a result of normal growth (4). Sex seems to be an important determinant of BMD, probably because of different muscle and sex steroid levels in boy and girls.
It should be noted that the cross sectional nature of this study limits the interpretation of our results, especially with regard to cause-effect interactions. Another limitation is the fact that other data about the life style of participants, such as calcium intake and exercise, were not evaluated.
In conclusion, in prepubertal girls, lean mass has a favorable effect on BMD. Fat mass seems not to protect the bone structure against osteoporosis, despite increased mechanical loading. Serum leptin may play a biological role in regulating bone metabolism. Further prospective studies including male and pubertal participants are necessary to apply our findings to the general population.
Figures and Tables
Table 1
Anthropometric characteristics, metabolic and biochemical parameters, body composition and bone mineral density of obese and control groups
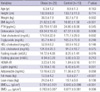
Table 3
Body mass index, serum fasting insulin, leptin, adiponectin levels and body composition as independent predictors of bone mineral density
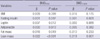
Results of multiple linear regression analyses including BMI, serum fasting insulin, leptin, adiponectin levels, fat and lean mass on bone mineral density. Unstandardized coefficients (β) and P values are presented.
BMDfemur, femoral bone mineral density; BMDL-spine, L-spine bone mineral density; BMI, body mass index.
References
1. Gascon F, Valle M, Martos R, Zafra M, Morales R, Castano MA. Childhood obesity and hormonal abnormalities associated with cancer risk. Eur J Cancer Prev. 2004. 13:193–197.
2. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004. 350:2362–2374.

3. Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002. 17:1563–1569.

4. Bouillon R, Prodonova A. Growth and hormone deficiency and peak bone mass. J Pediatr Endocrinol Metab. 2000. 13:Suppl 6. 1327–1336.
5. Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003. 32:78–85.

7. Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, Setoain J, Granados A. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int. 2001. 12:811–822.

8. Van Langendonck L, Claessens AL, Lefevre J, Thomis M, Philippaerts R, Delvaux K, Lysens R, Vanden Eynde B, Beunen G. Association between bone mineral density (DXA), body structure, and body composition in middle-aged men. Am J Hum Biol. 2002. 14:735–742.
9. Khosla S, Atkinson EJ, Riggs BL, Melton LJ 3rd. Relationship between body composition and bone mass in women. J Bone Miner Res. 1996. 11:857–863.

10. Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005. 37:474–481.

11. Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, Diaz R, Hernandez B, Del Cueto R, Hernandez-Avila M. Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int. 2003. 14:539–547.
12. Young D, Hopper JL, Macinnis RJ, Nowson CA, Hoang NH, Wark JD. Changes in body composition as determinants of longitudinal changes in bone mineral measures in 8 to 26-year-old female twins. Osteoporos Int. 2001. 12:506–515.

13. Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007. 92:143–147.

14. Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998. 83:3469–3475.

15. Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf). 2001. 55:341–347.

16. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001. 7:941–946.

17. Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006. 21:1648–1656.

18. Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005. 331:520–526.

19. Sugiyama T, Yamaguchi A, Kawai S. Effects of skeletal loading on bone mass and compensation mechanism in bone: a new insight into the "mechanostat" theory. J Bone Miner Metab. 2002. 20:196–200.

20. Glauber HS, Vollmer WM, Nevitt MC, Ensrud KE, Orwoll ES. Body weight versus body fat distribution, adiposity, and frame size as predictors of bone density. J Clin Endocrinol Metab. 1995. 80:1118–1123.

21. Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005. 36:568–576.

22. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002. 111:305–317.

23. Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001. 28:113–117.

24. Papadopoulou F, Krassas GE, Kalothetou C, Koliakos G, Constantinidis TC. Serum leptin values in relation to bone density and growth hormone-insulin like growth factors axis in healthy men. Arch Androl. 2004. 50:97–103.

25. Lorentzon M, Landin K, Mellstrom D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006. 21:1871–1878.

26. Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005. 309:99–109.

27. Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007. 92:1517–1523.

28. Huang KC, Cheng WC, Yen RF, Tsai KS, Tai TY, Yang WS. Lack of independent relationship between plasma adiponectin, leptin levels and bone density in nondiabetic female adolescents. Clin Endocrinol (Oxf). 2004. 61:204–208.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download