Abstract
We report a case of prenatally diagnosed congenital perineal mass which was combined with anorectal malformation. The mass was successfully treated with posterior sagittal anorectoplasty postnatally. On ultrasound examination at a gestational age of 23 weeks the fetal perineal mass were found on the right side. Any other defects were not visible on ultrasonography during whole gestation. Amniocentesis was performed to evaluate the fetal karyotyping and acetylcholinesterase which were also normal. As the fetus grew up, the mass size was slowly increased more and more. At birth, a female neonate had a perineal mass on the right side as expected. During operation, the anal sphincteric displacement was found near the mass and reconstructed through posterior sagittal incision. This is the first reported case of prenatally diagnosed congenital perineal mass, after birth which was diagnosed as lipoblastoma and even combined with anorectal malformation. This case shows that it can be of clinical importance to be aware of this rare fetal perineal mass in prenatal diagnosis and counseling.
Lipoblastoma is a rare benign tumor originated from adipose tissue and cytologically characterized by lipocyte, lipoblasts, spindle cells, myxoid extracellular material, thin branching capillaries, and absence of nuclear atypia (1-4). It is found most commonly in the upper and lower extremities but it can occur anywhere of soft tissue (5). It is usually noted during the first 3 yr of life and male predominant of 3:1 (6, 7). Spite of its benign nature, the rapid growth character generally makes the best treatment of choice complete surgical resection.
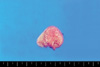
A 27-yr-old woman was referred for further evaluation of fetal perianal mass detected during routine antenatal care at 23 weeks' gestation. Nuchal translucency and quad marker tests were normal at the local clinic. At the first visit to our hospital, 0.9×0.81 cm sized freely movable, round shaped homogenous echogenic perivaginal mass with smooth surface was visible on two-dimensional (2D) transabdominal ultrasound (Accuvix XQ; Medison, Seoul, Korea). And, additional 3D ultrasound (Accuvix XQ) could reveal the origin of the mass which was originated from the skin apart from anus or vagina. The amniocentesis revealed the fetal karyotype was normal as 46, XX and acetylcholinesterase level was not detected. The fetal perivaginal mass was more and more increased to 1.32×0.96 cm at 31 weeks and 2.43×1.87 cm at 36 weeks as gestational age was increased (Fig. 1). A female neonate was delivered by induction with oxytocin at GA 41 weeks and weighted 3.63 kg. The Apgar score was 9 and 9 each at 1 and 5 min and the neonate was grossly normal. The pedunculated soft mass, measuring 3×2.1×2 cm, was visible on the right posterior labium major and the base of the mass was about 0.7 cm in diameter (Fig. 2). General physical and laboratory evaluation on the neonate was performed preoperatively. Complete blood counts, electrolytes, liver and renal function tests, and urinalysis were within normal range. Patent foramen ovale with a flow from left to right was visible in routine postnatal cardiac echo. Any abnormal pathogen was not detected in perineal culture and there was no specific finding on whole body radiography. Magnetic resonance imaging (MRI) study showed pedunculated fat mass in right perineum. In addition, there was no significant congenital anomaly in vertebral body, both kidney and anus on MRI. Abdominal ultrasound was also performed. On the ultrasound, the echotexture of the perineal pedunculated mass was similar with subcutaneous fat and there was no bowel content in it. Simple surgical excision was planned for the lesion originally. The mass was removed under general anesthesia (Fig. 3). At operation, we identified that the right anal external muscle complex was displaced due to the original mass and also found the perineal groove type anorectal malformation. Posterior sagittal anorectoplasty (PSARP) was performed with complete mass excision. The histopathologic result was lipoblastoma with free resection margin (Fig. 4). The neonate could be discharged from the hospital without any complication on the postoperative seventh day.
All of the reported cases of congenital perineal mass have been diagnosed after birth except for one case. Bord et al. (8) in 2006 reported a case of a congenital perineal skin tag that presented as a perineal tumor with 5 mm in diameter during second trimester sonographic scan at 23 weeks' gestation. It was lipoma histologically. Their report was the first prenatally diagnosed case of lesion on the fetal perineum. Since the first definition of a lipoblastoma to describe a tumor of immature fat cells by Jaffe in 1926 (9), some authors have reported lipoblastomas postnatally. In 1973, Chung and Enzinger (10) distinguished lipoblastomas of the circumscribed type from lipoblastomatosis of the diffuse, multicentric type, and identified the extremities as the most common site. A recent 16 case series by Jung et al. (5) reported the lesions involving 10 boys and 6 girls ranging from 5-49 months of age. In their report, the most common presenting symptom was a painless mass with a progressive increase in size, and the neck was the most common location. The tumor size varied from 2.0-12.0 cm in the greatest diameter, and the histologic results involved myxoid diffuse, circumscribed, or diffuse types with or without encapsulation (5). Our case is meaningful as the first report of a perineal lipoblastoma, which was prenatally diagnosed by 3D ultrasonography. It indicates that 2D and 3D ultrasound had an important role on the detection of origin and differential diagnosis of perineal mass and counseling with their parents. Although radiologic imaging can be helpful in differentiating soft tissue tumors, MRI and CT are of little use in a diagnosis of lipomatous tumors. Only increased cellularity of lipoblastoma makes lower density on T1-weighted MRI images and can be helpful in differentiating from lipoma (11).
Lipoblastoma is grossly soft and smooth mass and histologically well circumscribed and lobulated; the lobules are separated by thin fibroconnective tissue septa containing gaping, thick-walled blood vessels. Cytologically, the tumors are composed of uniform proliferation of slender spindle cells showing slightly eosinophilic cytoplasm with indistinct borders and uniform, elongated nuclei with finely granular chromatin, and no nucleoli (12). In cases of lipoblastomas, myxoid liposarcomas and lipomas should be ruled out because the histologic and cytological features are similar and occasionally indistinguishable (13). The clue for the differentiation of liposarcomas is the absence of nuclear atypia. The detection of a rearrangement of 8q11-q13 in lipoblastomas by chromosomal studies is another method for difficult cases; myxoid liposarcomas are characterized by the translocation t(12;16)(q13;p11) (14-16). The other differential diagnoses includes skin tags, hymenal tags, condylomas, hymenal and paraurethral cysts, vulvar hydroceles, capillary and cavernous hemangiomas, lymphangiomas, neurofibromas, embryonal rhabdomyosarcoma, teratoma, endodermal sinus tumor and lipomyelomeningocele (17).
Complete excision is important for treatment because there is a recurrence risk upto 14-25% (10, 18). After that, searching for anorectal malformation combined with perineal mass is also important. During excision of the neonatal perineal mass, we found anal sphincteric displacement near the mass and reconstructed the anal sphincter and perineal body through posterior sagittal incision similar to Shaul et al. (19). Anorectal malformation can have an effect on anorectal function. Wester and Rintala (20) insisted that the distortion of sphincter was the most important factor to decide the final anorectal functional result; in their cases, the sphincter was distorted in the majority of the patients. They concluded that it seems reasonable to inform parents of neonates with perineal lipomas associated with an anorectal malformation that the lipoma may have a negative impact to on the functional outcome. We agree to inform parents of the neonates that their offsprings can have incontinent problem in their later lives. Shaul et al. (19) showed that hamartomas/choristomas, vascular anomalies, such as hemangiomas, lymphaticovenous malformations, and lipomas could be associated with anorectal malformations.
Although fetal perineal mass is rare, it can be detected by careful prenatal ultrasonography. And the 3D ultrasound images make it possible to differentiate the origin of mass which is derived from anus, vagina, vulva, etc. If the perineal mass is found during antenatal care, general counseling on diagnosis, treatment, and prognosis of the mass should be given to parents of the fetuses. Also, it is necessary to perform a precise evaluation of the fetal pelvic anatomy i.e. anal canal, vagina, uterus and bladder, to rule out a more complex pelvic malformation. The patients with anorectal malformations may have cloacas, rectovestibular fistulas, and sacral anomalies, such as a hemisacrum and meningoceles (20).
We first report prenatally detected congenital perineal mass by ultrasound which was diagnosed as lipoblastoma combined with anorectal malformation after birth.
Figures and Tables
 | Fig. 1(A) 2D and (B) 3D ultrasonographic findings of fetal perineal mass (arrow) with hypoechoic shadow at 36 weeks' gestation. |
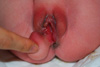 | Fig. 2At birth, pedunculated round soft mass, measuring 3×2.1×2 cm, was visible on the right posterior labium major of the newborn. |
References
1. Talwar MB, Misra K, Marya SK, Dev G. Fine needle aspiration cytology of a lipoblastoma. Acta Cytol. 1990. 34:855–857.
2. Lopez-Ferrer P, Jimenez-Heffernan JA, Yebenes L, Vicandi B, Viguer JM. Fine-needle aspiration cytology of lipoblastoma: a report of two cases. Diagn Cytopathol. 2005. 32:32–34.
3. Walaas L, Kindblom LG. Lipomatous tumors: a correlative cytologic and histologic study of 27 tumors examined by fine needle aspiration cytology. Hum Pathol. 1985. 16:6–18.

4. Pollono DG, Tomarchio S, Drut R, Zaritzky M, Otero L, Vazquez AJ, Ripoll MC. Retroperitoneal and deep-seated lipoblastoma: diagnosis by ct scan and fine-needle aspiration biopsy. Diagn Cytopathol. 1999. 20:295–297.

5. Jung SM, Chang PY, Luo CC, Huang CS, Lai JY, Hsueh C. Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 16 cases in Taiwan. Pediatr Surg Int. 2005. 21:809–812.

6. Coffin CM. Lipoblastoma: an embryonal tumor of soft tissue related to organogenesis. Semin Diagn Pathol. 1994. 11:98–103.
7. Stringel G, Shandling B, Mancer K, Ein SH. Lipoblastoma in infants and children. J Pediatr Surg. 1982. 17:277–280.

8. Bord A, Valsky DV, Yagel S. Prenatal sonographic diagnosis of congenital perineal skin tag: case report and review of the literature. Prenat Diagn. 2006. 26:1065–1067.

9. Jaffe RH. Recurrent lipomatous tumors of the groin: liposarcoma and lipoma pseudomyxomatodes. Arch Pathol. 1926. 1:381–387.
10. Chung EB, Enzinger FM. Benign lipoblastomatosis. An analysis of 35 cases. Cancer. 1973. 32:482–492.
11. Ha TV, Kleinman PK, Fraire A, Spevak MR, Nimkin K, Cohen IT, Hirsh M, Walton R. Mr imaging of benign fatty tumors in children: report of four cases and review of the literature. Skeletal Radiol. 1994. 23:361–367.

12. Lae ME, Pereira PF, Keeney GL, Nascimento AG. Lipoblastoma-like tumour of the vulva: report of three cases of a distinctive mesenchymal neoplasm of adipocytic differentiation. Histopathology. 2002. 40:505–509.

13. Mentzel T, Calonje E, Fletcher CDM. Lipoblastoma and lipoblastomatosis: a clinicopathological study of 14 cases. Histopathology. 1993. 23:527–533.

14. Dal Chin P, Sciot R, De Wever I, Van Damme B, Van den Berghe H. New discriminative chromosomal marker in adipose tissue tumors. The chromosome 8q11-q13 region in lipoblastoma. Cancer Genet Cytogenet. 1994. 78:232–235.
15. Miller GG, Yanchar NL, Magee JF, Blair GK. Tumor Karyotype differentiates lipoblastoma from liposarcoma. J Pediatr Surg. 1997. 32:1771–1772.

16. Tallini G, Akerman M, Dal Cin P, De Wever I, Fletcher CD, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Sciot R, Van den Berghe H, Van den Ven W, Vanni R, Willen H. Combined morphologic and karyotypic study of 28 myxoid liposarcomas. Implications for a revised morphologic typing, (a report from the CHAMP Group).Implication of a revised morphologic typing (A report from the CHMP group). Am J Surg Pathol. 1996. 20:1047–1055.
17. Ambros RA, Kurman RJ. Rock JA, Carpenter SE, editors. Tumors of the vulva. Pediatric and adolescent gynecology. 1992. New York: Raven Press;353–363.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download