INTRODUCTION
Primary aldosteronism (PA) is characterized by the inappropriate excess production of aldosterone, regulated in part independently of the renin-angiotensin system. PA is commonly caused by aldosterone-producing adenoma (APA) or bilateral adrenal hyperplasia, also known as idiopathic hyperaldosteronism (IHA). PA causes hypertension, hypokalemia, and alkalosis. PA induced by APA is one of the few potentially curable causes of hypertension and affects 1.4% to 10% of hypertensive patients (1-5).
Adrenalectomy for APA is the ideal treatment to relieve hypokalemia and improve blood pressure control. Although hypokalemia resolves in more than 98% of patients after adrenalectomy for an APA, hypertension resolves completely in only 20% to 35% without further hypertension medications (6-10). This condition is called resistant hypertension, of which exact cause is unknown.
Recently, studies have suggested that resistant hypertension is associated with dysregulation of the cardiovascular system in APA (11-14). Mechanisms of disease pathology include chronically high levels of aldosterone-which causes chronic intravascular fluid retention-arterial stiffness, suppression of endothelial function, induction of target organ inflammation, and fibrosis (15). The benefits of adrenalectomy on APA of the adrenal gland in hypertension resolution, however, are debated.
The aim of this paper was to investigate the clinical behaviors and treatment outcomes in APA after adrenalectomy and to identify the preoperative clinical predictors that influence the outcomes of hypertension.
MATERIALS AND METHODS
This study is a retrospective case series of patients who underwent surgical intervention for APA at Ajou University Hospital from December 1995 to September 2008. Thirty-eight patients with PA entered into the database. Due to missing data or inconsistencies regarding the diagnosis and lack of surgical intervention, 11 patients were initially excluded from further analysis. The remaining 27 PA patients were correlated with this study subjects. We received IRB approval and the recognition number is AJIRB-MED-MDB-09-217.
Extensive clinical data were extracted from patients' charts, including laboratory test results, dynamic testing, medication, adrenal imaging, details of specific medical treatment, and surgical treatment. We checked blood pressure at admission, postoperatively, and during the follow-up, and we examined the preoperative clinical variables that associated most strongly with resolution of hypertension postoperatively to design a multivariate predictive model.
The following baseline variables were obtained through a review of medical records: age, body mass index (BMI), tumor size, family history of hypertension, number of antihypertensive medications, and duration of hypertension. Family history of hypertension was defined as having one or more first-degree family members with documented hypertension. The laboratory data that were collected included blood levels of potassium and aldosterone, plasma renin activity, and aldosterone-to-plasma renin activity ratios.
We assessed the degree of improvement in hypertension after adrenalectomy and the clinical outcome data, which consisted of systolic blood pressure (SBP), diastolic pressure (DBP) and the number of antihypertensive medications at the postoperative visit. We categorized patients into 3 groups; clinically cured, improved control, and resistance groups. Patients were labeled as clinically cured if they had no hypertension, defined as SBP <140 mmHg and DBP M <90 mmHg, and were not taking any antihypertensive medications after surgery.
Patients were categorized as having improved control of their blood pressure if they were normotensive and required an equal number of or fewer antihypertensives postoperatively, or were hypertensive and required fewer antihypertensives.
Subjects were placed into the resistant hypertension group if either of 2 criteria were met 6 months postoperatively; persistent hypertension (defined as SBP ≥140 mmHg or DBP ≥90 mmHg), and continued need of antihypertensive medications to control blood pressure adequately. We also compared preoperative clinical factors between groups to predict postoperative hypertension resolution outcomes according to these criteria. The mean follow-up period was 51.8±47.0 months.
In the univariate analyses, we compared each of these variables using t-tests, chi-square tests, and ANOVA, when appropriate. To obtain a simple and practical model, continuous variables were dichotomized using rounded, whole-number values that allowed effective discrimination between the groups. We selected these cutoffs by identifying predictor variable values on a receiver operating characteristic (ROC) curve. After the univariate analysis, a multivariate logistic regression model of patient characteristics and outcomes was performed to identify the clinical variables that were associated with complete resolution. We recognized the results, when P value is less than 0.01.
RESULTS
From December 1995 to September 2008, 27 patients underwent surgery for adenoma-induced primary aldosteronism. The mean age of the patients was 45.3±4.0 yr, and the male-to-female ratio was 1:2. The chief symptoms that were used to establish the diagnosis of PA included a history of persistent hypertension (85.1%), hypokalemic alkalosis (92.5%), muscle weakness (25.9%), paresthesia (7.4%), and paralysis (11.1%). Five patients (18.5%) had a family history of hypertension. Seventeen patients underwent laparoscopic surgery, and 10 patients underwent open surgery. Eighteen patients had the lesion on the right side, and 9 had it on the left. There were no serious postoperative complications.
The mean duration of hypertension was 67.5±94.0 months (range 2.0-408.0 months), and the mean number of antihypertensive medications was 2.3±1.0 (range 1-6). The most commonly used antihypertensive medications were beta-blockers, alpha-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors. Mean body mass index was 23.8±3.0 kg/m2 (range 16.6-28.8 kg/m2), mean preoperative serum aldosterone level was 124.3±36.0 pg/mL (range 66.0-1,915.0 pg/mL), and mean serum renin was 0.5±0.8 ng/mL (range 0.1-4.0 pg/mL). The mean aldosterone-renin ratio was 252.2±28.0 (range 7.9-4,905.0). All patients had diagnosed adenomas, and the mean tumor size was 2.2±1.0 cm (range 1.0-5.0 cm). Mean preoperative blood pressure was 162.8±12.0/99.4±18.0 mmHg (range 123-200/124-53 mmHg), and mean potassium level was 2.5±0.6 mM (range 1.5-4.3 mM).
Hypokalemia was resolved after adrenalectomy in all patients at postoperative Week 1, wherein the mean was 4.1±0.5 mM (range 3.0-5.7 mM). Hypertension was completely resolved only in 16 patients (59.2%) postoperatively, however; the mean blood pressure was 130.5±12.0/82.8±11.0 mmHg (range 107-154/66-105 mmHg). The duration of resolution was 5.7±4.0 months (range 1.0-5.0 months). According to our category criteria, 16 (59.2%) were in clinically cured group, 7 (25.9%) had improved control, and 4 (14.8%) experienced an ongoing need for medications and had worse blood pressure control (resistance group). The mean number of postoperative antihypertensive medications was 1.0±0.5.
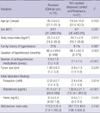
In this study, age, gender, BMI, tumor size, and preoperative serum potassium and renin levels were not significantly associated with complete resolution. In contrast, other variables that were significantly associated with complete resolution of hypertension included antihypertensive medications, duration of hypertension, and plasma aldosterone concentrations (Table 1). When the proper cutoffs for variables were selected by analyzing the ROC curve for each variable, the number of antihypertensive medications (≤2), duration of hypertension (≤6 yr), and plasma aldosterone levels (≤350 pg/mL) were included in the multivariate analysis. On multivariate analysis, serum aldosterone level (≤350 pg/mL) was the only predictor that correlated with complete resolution of hypertension (P<0.001) (Table 2, Fig. 1).
DISCUSSION
As first described by Conn in 1955, PA was thought to be an atypical cause of hypertension (16), which had a prevalence of <1% among general hypertensive patients (16, 17). Recent studies indicate, however, that PA is much more common than originally reported and have estimated prevalence more than 10% of the general hypertensive population (18) and approximately 20% of patients with severe or persistent hypertension (5,14). PA is caused by APA in 30% to 60% of cases (9, 21), occurs more frequently in women than in men, and occurs rarely in children (22). We observed a male-to-female ratio of 1:2, consistent with the previous studies.
The clinical features of APA are nonspecific. Some patients are asymptomatic or have minimal symptoms. Here, the clinical manifestations of APA included hypertension, hypokalemic alkalosis, renal dysfunction, nephrogenic diabetes insipidus, muscle weakness, paresthesia, tetany, and paralysis (5, 14-17). In our study, the most common clinical manifestation that was used to establish a diagnosis of PA was a history of persistent hypokalemic alkalosis (92.5%). Hypertension (85.1%), muscle weakness (25.9%), paresthesia (7.4%), and paralysis (11.1%) also were found.
Adrenalectomy typically is chosen to resolve hypokalemia and improve blood pressure control in APA patients. Many researchers have suggested that hypokalemia is corrected in more than 90% of patients with APA postoperatively (6-10).
Resistant hypertension, however, defined as blood pressure that remains elevated despite antihypertensive agent use, is frequently observed. Although studies have noted the prevalence of resistant hypertension after adrenalectomy, the estimates of its prevalence vary widely, from 9% to 65% (6, 8, 9, 13, 17, 20). This pattern is due to the fact that most studies have been performed retrospectively in a selected cohort of patients and have used heterogeneous criteria for selecting patients. In our study, complete resolution of hypertension was observed in 59.2% of patients, and control of hypertension improved in 25.9% of patients. Resistant hypertension was found in only 14.8% of subjects.
Some authors have opined that the mechanism that induces resistant hypertension relies on multiple reactions after long-term aldosterone excess in cardiovascular and renovascular system (11-14, 23). They also suggested that high level of aldosterone impairs vascular function by suppression of nitric oxide formation and increases in reactive oxygen species. Others have shown that inappropriate high aldosterone levels with regard to sodium status cause extensive renal damage (24, 25). Aldosterone excess is also associated with glomerular hyperfiltration and declines in glomerular filtration rate (24, 25). A recent study has emphasized chronic intravascular fluid retention, arterial stiffness, suppression of endothelial function, and induction of target organ inflammation and fibrosis in resistant hypertension (15). The underlying cause of resistant hypertension, however, is controversial.
The clinical factors that predict the resolution of hypertension in APA after adrenalectomy have been investigated and evaluated often, but the precise factors remain unknown. Other studies have presented results that have differed from ours, suggesting that younger age, smaller tumor, lower urinary aldosteronerenin ratio, lower BMI, and lack of family history of hypertension are predictors of resolution of hypertension after adrenalectomy (8, 15, 25, 26).
A recent study that used aldosterone resolution score (ARS) to determine score found female gender, use of ≤2 antihypertensive medications, BMI <25, and <6 yr of hypertension to be good predictors (6). In our study, plasma aldosterone levels (<350 pg/mL), duration of hypertension (<6 yr), and number of antihypertensives (≤2) were adequate predictors of surgical curability of APA, based on our univariate analysis. In our multivariate analysis, however, plasma aldosterone level was the only true useful predictor of adrenalectomy; plasma aldosterone level (<350 pg/mL) was the most accurate independent predictor of the effectiveness of surgery.
Although the retrospective nature of our study has limitations, and although the mechanism of resistant hypertension is unclear, these predictors can help clinicians provide objective information about treatment outcomes to patients with APA before they undergo surgical intervention. In the future, if we obtain data on more patients and perform the study prospectively, we can determine the exact mechanism of resistant hypertension and identify more accurate predictors.
In conclusion, our study shows that 59.2% of patients experienced complete resolution of hypertension and that control of hypertension improved in 25.9% of patients. Resistant hypertension occurred in 14.8% of patients. The best preoperative clinical factor that predicted postoperative hypertension resolution after adrenalectomy is serum aldosterone level (<350 pg/mL).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download