Abstract
Radical nephrectomy with inferior vena cava (IVC) thrombectomy remains the most effective therapeutic option in patients with renal cell carcinoma and IVC tumor thrombus. Cephalic extension of the thrombus is closely related to perioperative morbidity. We purposed to design a safe and successful surgical strategy through a review of our surgical experience and treatment results in 35 patients (male:female=28:7, mean age=56 yr [32-77]) who underwent IVC thrombectomy with radical nephrectomy between January 1997 and December 2006. The limit of tumor extension was level I in 10 patients (28.6%), level II in 17 (48.6%), and level III and IV in 4 patients each (11.4%). Liver mobilization with hepatic vascular exclusion was performed in 12 patients and cardiopulmonary bypass in 7. Thirty-two primary closures, 2 patch closures, and 1 graft interposition were performed. One patient underwent simultaneous pulmonary embolectomy because of an operative pulmonary embolism. There was no operative mortality, and the overall survival at 5-yr was 50.8%. Complete thrombus removal without tumor fragmentation under long venotomy on fully exposed involved IVC is recommended for successful result in a bloodless operative field. The applicability of liver mobilization, hepatic vascular exclusion, and cardiopulmonary bypass, can be determined by the level of thrombus.
Involvement of the inferior vena cava (IVC) has been reported in up to 35% of patients with renal cell carcinoma (RCC) (1-6). Although complete removal of the extension of the tumor thrombus into the IVC does not affect patient prognosis (7, 8), non-removal has been associated with poor survival rates (7). The most effective therapeutic option in patients with RCC and IVC tumor thrombus is aggressive surgical resection, including radical nephrectomy with IVC thrombectomy, even in patients with distant metastases (8-11). Five-year survival rates following surgery have been reported to range from 32% to 64% (2, 6, 7, 9).
Even with improvements in preoperative diagnostic modalities, anesthesiology, and perioperative care, there is still considerable morbidity and mortality in this type of surgery, ranging from 2.7% to 40% (3, 8, 12), arising primarily from massive pulmonary embolism and hemorrhage (7, 13). These complications are closely related to the cephalic extension of the IVC tumor thrombus, suggesting that radical surgery should be reserved for patients with tumor thrombi extending beyond the level of the diaphragm (12). We have designed a safe and successful surgical strategy through a review of our surgical experience.
From January 1997 to December 2006, 35 patients at the Asan Medical Center, Seoul, Korea, underwent IVC tumor thrombectomy with radical nephrectomy, performed by urological and vascular surgeons. Preoperative assessment of distant metastases and extents of thrombus was determined mainly by computed tomography (CT) of the thorax, abdomen, and pelvis. Nineteen patients underwent magnetic resonance image (MRI). After discharge, patients were followed up every 3 months and CT scans were performed every 6 months.
Tumors were classified according to the TNM classification of the UICC International Union against Cancer (IUAC) (14). Extent of the thrombus was graded at level I or renal (i.e., extending ≤2 cm above the ostium of the renal vein into the IVC); level II or infrahepatic (i.e., extending >2 cm above the ostium of the renal vein and below the intrahepatic vena cava); level III or intrahepatic (i.e., extending into the intrahepatic portion of the vena cava, but below the diaphragm); or level IV or atrial (i.e., extending into the right atrium) (9).
Statistical analysis was performed using the statistical package SPSS for Windows (Version 12.0, SPSS Inc., Chicago, IL, USA). Statistical significance was defined when a P value of <0.05 was obtained. Overall survival rate was calculated using the Kaplan-Meier method.
Under general anesthesia, a central venous line and radial artery catheter were placed to allow fluid administration and blood pressure monitoring. Intraoperative continuous TEE surveillance was used to detect tumor or gas emboli and to confirm complete thrombus removal.
Laparotomy was usually performed using an inverted T incision or a chevron incision, both of which can be extended to the thorax when cardiopulmonary bypass (CPB) is required. The colon was retracted medially and the parietal peritoneum was incised over the kidney near the colon. In patients who did not undergo preoperative renal artery embolization, the renal artery was ligated first. The colon was reflected medially to expose the IVC and aorta. The IVC was freed from the lumbar veins, both renal veins were exposed, and slings were passed around these structures, taking care not to dislodge the thrombus. Following administration of heparin, clamps were applied successively to the distal IVC, the renal vein contralateral to the tumor, and the upper IVC proximal to the thrombus. A lateral venotomy was performed from the ostium of the tumoral renal vein to the IVC beyond the proximal limit of the thrombus and the thrombus was completely removed under direct vision (Fig. 1A, B). We avoided blind digital extraction, milking, or Fogarty thrombectomy of IVC thrombus. The ostium of renal vein was sent for frozen biopsy. Primary repair of the cavotomy was done if the margin was free of the tumor, and when gross invasion into the IVC wall was present cavectomy with prosthetic graft interposition or patch angioplasty was performed (Fig. 1C). After closure of the IVC, radical nephrectomy was performed. Following surgery, patients were transferred to the intensive care unit. Anticoagulation therapy, using low molecular-weight heparin followed by antiplatelet agent(s), was administered for 6 months after the procedure.
Operative maneuvers based on the limit of thrombus extension are summarized at Table 1.
Liver mobilization was individualized depending on thrombus extent. If preoperative imaging showed sufficient space to apply a proximal venous clamp, exposure of the IVC was limited to the infrahepatic portion. Venous clamps were successively positioned on the infrarenal IVC, the renal vein contralateral to the tumor, and the IVC between the origin of the hepatic veins and the limit of thrombus extension.
The liver was mobilized completely by the piggyback technique, dividing the falciform, triangular, and coronary ligaments, and the IVC was completely dissected from the renal vein level to the hepatic veins. After complete hepatic mobilization and IVC cross-clamping, hepatic vascular exclusion was performed to prevent bleeding from the hepatic vein and hepatic congestion. The suprahepatic IVC, the infrarenal IVC, the contralateral renal vein, and the hepatic vein were occluded in sequence. Hepatic venous thrombi were removed with a Fogarty catheter
Laparotomy was associated with sternotomy for CPB. CPB was started after cannulation of the aorta, superior vena cava, and right femoral vein or infrarenal IVC. Deep hypothermia was usually not used. After making a long venotomy below the diaphragm, a right atriotomy for tumor mobilization and extraction was performed. Complete removal of the thrombus was confirmed by inspecting and palpating the right atriumand the wall of IVC.
The 35 patients consisted of 28 men and 7 women, of mean age 56 yr (range, 32-77 yr). Eleven patients had tumors in the right kidney and 24 in the left kidney. Median follow up period was 28 months (range, 2-80 months). Preoperative renal artery embolization was performed in 10 patients.
Based on histological findings, the tumors were classified as pT3b in 22 patients (62.9%), pT3c in 6 (17.1%), and pT4 in 7 (20.0%) (Table 2). Pathological examination revealed 28 clear cell carcinomas (80.0%), 5 papillary cell carcinomas (14.3%), 1 sarcomatoid carcinoma (2.9%), and 1 chromophobe carcinoma (2.9%). Four patients (11.4%) had lymph node metastases and 8 (22.9%) had distant metastases at the time of diagnosis.
The limit of tumor extension was level I in 10 patients (28.6%), level II in 17 (48.6%), level III in 4 (11.4%), and level IV in 4 (11.4%) (Table 2). Four patients had concomitant iliac vein thrombosis.
Right liver mobilization with hepatic vascular exclusion was performed in 12 patients, 4 of 17 with level II thrombi, 4 of 4 with level III and 4 of 4 with level IV. CPB was used in 7 patients, including 2 with level III thrombi, 4 with intraatrial thrombi and 1 with intraoperative pulmonary embolism.
After thrombectomy, primary closure was possible in 32 patients (91.4%) without significant diameter loss, because of dilatation of the IVC. Because of involvement of the IVC wall, 2 patients required patch closure and 1 required graft interposition (Table 2). Except three patients with recurrent tumor of the IVC, all patients showed good patency of the IVC (Fig. 2).
There was no operative mortality or hepatic insufficiency. However, simultaneous pulmonary embolectomy was performed in 2 patients, both with infrahepatic thrombi, because of operative pulmonary embolism during dissection and manipulation of the kidney and IVC. Patients with level III and IV thrombosis required larger amount of mean blood transfusion compared to patients with level I and II (14.8 vs. 5.9 packs, P<0.05). Operation time was significantly longer in patients with level III and IV thrombosis than level I and II (594.0 vs. 325.7 min, P<0.05). Likewise, postoperative intensive care unit stay was longer (3.8 vs. 1.7 days, P<0.05).
The overall 5-yr survival rate was 50.6% and median survival was 54 months (Fig. 3A). Seventeen patients presented with tumor recurrence, most commonly in the lungs (58.8%). There were 11 (31.4%) late deaths during the follow-up period. The 2-yr survival rate of patients with renal, infrahepatic, intrahepatic, and atrial tumor extension was 90.0%, 79.5%, 66.7%, and 50.0%, respectively (Fig. 3B). The 2-yr survival rate of stage 3 and stage 4 was 82.5% and 64.3% (Fig. 3C). Extent of tumor thrombus was not significantly associated with survival, nor was stage, lymph node involvement, visceral involvement, or invasion of the IVC wall (P>0.05 for each association).
Perioperative IVC filters were used in 8 patients, including preoperative IVC filters in 3, intraoperative in 3, and postoperative in 2. Four IVC filters were inserted for preventing pulmonary embolism, 2 after pulmonary embolectomy, and 2 with pulmonary embolisms during follow-up. Intraoperative IVC filter placement was performed through femoral vein or internal jugular vein after IVC repair. One IVC filter which was inserted prophylactically, was retrieved during surgery because it hindered proximal venous clamp. No postoperative IVC occlusion was related to IVC filter use during follow-up period.
Treatment strategies of RCC has continued to evolve from understanding of molecular and genetic characteristics of this disease (15). However, patients with RCC and IVC thrombosis have major concern on immediate postoperative complication mainly from massive pulmonary embolism and bleeding during surgery before getting benefit on outcome with adjuvant therapies (1, 6, 7, 9). To proceed safe and successful operation regarding IVC thrombectomy, first of all, intensive intraoperative hemodynamic monitoring was required. We found that patients with intraoperative pulmonary embolisms showed sudden decreases in O2 saturation and blood pressure, suggesting a need for continuous surveillance with intraoperative bidimensional TEE. TEE is useful in the preoperative evaluation of thrombus extent, in detecting tumor or gas emboli, and in intraoperative confirmation of complete thrombus removal (6, 16, 17). In our experience, intraoperative pulmonary embolism developed during dissection and manipulation of the IVC and kidney, and we recommend using TEE routinely at every stage of the procedure.
Pulmonary embolisms can be prevented by sufficient exposure of the IVC for proximal control and direct inspection of involved IVC wall. Complete thrombus extraction without tumor fragmentation can be achieved through liver mobilization using liver transplantation techniques (18-22). Piggyback liver mobilization can ensure direct visualization of the entire length of the IVC, thus permitting a cavotomy to be extended when necessary. Reported complications of liver mobilization include duodenal injury, liver laceration, and pulmonary embolism, suggesting that liver mobilization be limited to level III and IV tumor extensions and selected patients with level II extensions (13, 18, 21). These surgical strategies also related with the long term outcome. Blind removal of a thrombus can cause postoperative embolisms and early disease recurrence because of tumor fragmentation and the intraluminal persistence of part of a thrombus (21). Patients with incomplete resection have a significantly worse prognosis, and their five-year survival rates following surgery have been reported to range from 0% to 17.5% (2, 7, 9). Our overall survival rate of 50.8% at 5-yr was similar to other reports of tumor resection through long cavotomy under full exposure of the involved IVC (3, 6).
We found that lateral venotomy, starting from the ostium of the affected renal vein, was convenient to effect repairs. In case of tumor invasion of the caval wall, medial venotomy carries a higher risk of narrowing after caval wall excision and renal vein repair, and often requires caval wall reconstruction.
Other reported strategies that may prevent pulmonary embolism include suprarenal IVC control using suprarenal IVC clips, sternotomy with direct clamping, IVC interruption by suture/staple ligation, and placement of a suprarenal IVC filter (23-27). Good technical success has been seen after various types of filters were used, with venography at the time of filter placement providing a preoperative assessment of the extent of the tumor thrombus (26). Sosa et al. (28) reported that preoperatively placed Mobin-Uddin vena caval umbrellas trapped tumor thrombi and/or clots in 5 of 7 patients. Intraoperative deployment of a filter may protect the propagation of bland thrombi in patients with distal thrombi as well as tumor thrombi (27). In contrast, the overall rate of vena caval thrombosis or occlusion and renal dysfunction associated with infrarenal filter placement was 3-5%, suggesting that filter placement may increase significant overall morbidity in the setting of recent nephrectomy (24). Moreover, incorporation of the tumor in the filter may increase the difficulty of complete resection (27). In our experience, use of preoperative IVC filters granted little room for applying venous clamps and we had to remove one during operation. Because direct inspection of involved IVC wall alone might decrease chance of pulmonary embolism, we do not recommend the prophylactic use of IVC filters. If IVC filter is required, careful selection of the IVC filter location is crucial, based on the extent of dissection.
Cardiopulmonary bypass or Pringle's maneuver may minimize bleeding from the operative field, thus achieving hemodynamic stability (13, 19, 29). CPB with or without hypothermia and circulatory arrest may achieve stable hemodynamic status during clamping of the IVC, thus providing a bloodless operative field for tumor resection and venous reconstruction in patients with level III or IV thrombi. However, half of IVC thrombi are infrahepatic and only 10% are located in the right atrium (11). Generally, CPB is the main cause of a complex systemic inflammatory response, which significantly contributes to several adverse postoperative outcomes, including renal, pulmonary, and neurological complications, bleeding, and even multiple organ dysfunctions (5, 30). Postoperative bleeding and coagulation disturbances were the most frequently reported complications in patients who underwent IVC thrombectomy with nephrectomy. Our findings suggest that CPB is not always necessary to achieve nephrectomy with thrombectomy of the IVC in patients without intraatrial thrombi. We usually use CPB without hypothermia or circulatory arrest. Furthermore, when infrarenal IVC is confirmed free from thrombus, we cannulate the infrarenal IVC instead of the femoral vein. This does not require further dissection of the femoral vein and may facilitate IVC manipulation, reducing bleeding from the IVC and assuring venous drainage of the lower extremities. Oblique application of a vascular clamp across the infrarenal IVC and contralateral renal vein can also assure renal venous drainage.
Another reported surgical technique that may achieve hemodynamic stability is aortic cross-clamping during IVC thrombectomy. Partial or total cross-clamping of the infrarenal or supraceliac segment of the abdominal aorta can maintain systemic blood pressure above 100 mmHg by maintaining venous return (13). However, in our experience, even patients without venous occlusion tolerated this procedure without significant hemodynamic instability, and transient hypotension could be managed by fluid and blood administration.
The role of anticoagulation after operation for preventing recurrent IVC thrombosis is not well described in many articles. The brief use of low molecular weight heparin or dextran was reported during the immediate postoperative period to prevent deep vein thrombosis (19, 26).
In conclusion, our findings suggest that safe IVC thrombectomy can be accomplished by vigilance on hemodynamic status and by complete thrombus removal without fragmentation through long venotomy on fully exposed involved IVC. Individual surgical planning is necessary, including liver mobilization, hepatic vascular exclusion, and possible CPB, depending on the level of thrombus.
Figures and Tables
Fig. 1
Operative pictures. After full exposure of the involved segment of the inferior vena cava (IVC), upper margin of thrombus (A) and presence of remaining thrombus attached to the venous wall is identified under direct vision (B). Since invasion into the vein often starts from renal vein level, the venotomy site might be successfully repaired without narrowing in case of resection of IVC wall when incision was extended from renal vein ostium (C).
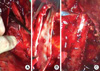
Fig. 2
Computed tomography of the patients with level IV thrombosis. Thrombus is extended to the suprahepatic IVC (A) and right atrium (B). Coronal reconstruction view demonstrates IVC thrombus extended from left renal vein to the right atrium (C).

References
1. Kearney GP, Waters WB, Klein LA, Richie JP, Gittes RF. Results of inferior vena cava resection for renal cell carcinoma. J Urol. 1981. 125:769–773.

2. Hatcher PA, Anderson EE, Paulson DF, Carson CC, Robertson JE. Surgical management and prognosis of renal cell carcinoma invading the vena cava. J Urol. 1991. 145:20–23.

3. Nesbitt JC, Soltero ER, Dinney CP, Walsh GL, Schrump DS, Swanson DA, Pisters LL, Willis KD, Putnam JB Jr. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Ann Thorac Surg. 1997. 63:1592–1600.

4. Tsuji Y, Goto A, Hara I, Ataka K, Yamashita C, Okita Y, Kamidono S. Renal cell carcinoma with extension of tumor thrombus into the vena cava: surgical strategy and prognosis. J Vasc Surg. 2001. 33:789–796.

5. Bissada NK, Yakout HH, Babanouri A, Elsalamony T, Fahmy W, Gunham M, Hull GW, Chaudhary UB. Long-term experience with management of renal cell carcinoma involving the inferior vena cava. Urology. 2003. 61:89–92.

6. Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004. 94:33–41.

7. Skinner DG, Pritchett TR, Lieskovsky G, Boyd SD, Stiles QR. Vena caval involvement by renal cell carcinoma. Surgical resection provides meaningful long-term survival. Ann Surg. 1989. 210:387–392.
8. Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol. 2004. 171:598–601.

9. Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987. 59:390–395.

10. Haferkamp A, Bastian PJ, Jakobi H, Pritsch M, Pfitzenmaier J, Albers P, Hallscheidt P, Muller SC, Hohenfellner M. Renal cell carcinoma with tumor thrombus extension into the vena cava: prospective long-term followup. J Urol. 2007. 177:1703–1708.

11. Kirkali Z, Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol. 2007. 52:658–662.

12. Staehler G, Brkovic D. The role of radical surgery for renal cell carcinoma with extension into the vena cava. J Urol. 2000. 163:1671–1675.

13. Jibiki M, Iwai T, Inoue Y, Sugano N, Kihara K, Hyochi N, Sunamori M. Surgical strategy for treating renal cell carcinoma with thrombus extending into the inferior vena cava. J Vasc Surg. 2004. 39:829–835.

14. Sobin LH, Wittekind CH. UICC International Union Against Cancer. TNM classificatoin of malignant tumors. 2002. 6th ed. New York: Wiley-Liss;193–195.
16. Oikawa T, Shimazui T, Johraku A, Kihara S, Tsukamoto S, Miyanaga N, Hattori K, Kawai K, Uchida K, Takeshima H, Saito S, Toyooka H, Akaza H. Intraoperative transesophageal echocardiography for inferior vena caval tumor thrombus in renal cell carcinoma. Int J Urol. 2004. 11:189–192.

17. Zini L, Haulon S, Decoene C, Amara N, Villers A, Biserte J, Leroy X, Koussa M. Renal cell carcinoma associated with tumor thrombus in the inferior vena cava: surgical strategies. Ann Vasc Surg. 2005. 19:522–528.

18. Kaplan S, Ekici S, Dogan R, Demircin M, Ozen H, Pasaoglu I. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Am J Surg. 2002. 183:292–299.

19. Vaidya A, Ciancio G, Soloway M. Surgical techniques for treating a renal neoplasm invading the inferior vena cava. J Urol. 2003. 169:435–444.

20. Delis S, Dervenis C, Lytras D, Avgerinos C, Soloway M, Ciancio G. Liver transplantation techniques with preservation of the natural venovenous bypass: effect on surgical resection of renal cell carcinoma invading the inferior vena cava. World J Surg. 2004. 28:614–619.

21. Gallucci M, Borzomati D, Flammia G, Alcini A, Albino G, Caricato M, Esposito A, Vincenzi B, Rossi M, Coppola R, Berloco P. Liver harvesting surgical technique for the treatment of retro-hepatic caval thrombosis concomitant to renal cell carcinoma: perioperative and long-term results in 15 patients without mortality. Eur Urol. 2004. 45:194–202.

22. Ciancio G, Livingstone AS, Soloway M. Surgical management of renal cell carcinoma with tumor thrombus in the renal and inferior vena cava: the University of Miami experience in using liver transplantation techniques. Eur Urol. 2007. 51:988–994.

23. Rosenthal D, Gershon CR, Rudderman R. Renal cell carcinoma invading the inferior vena cava: the use of the Greenfield filter to prevent tumor emboli during nephrectomy. J Urol. 1985. 134:126–127.

24. Brenner DW, Brenner CJ, Scott J, Wehberg K, Granger JP, Schellhammer PF. Suprarenal Greenfield filter placement to prevent pulmonary embolus in patients with vena caval tumor thrombi. J Urol. 1992. 147:19–23.

25. Hirota S, Matsumoto S, Ichikawa S, Tomita M, Koshino T, Sako M, Kono M. Suprarenal inferior vena cava filter placement prior to transcatheter arterial embolization (TAE) of a renal cell carcinoma with large renal vein tumor thrombus: prevention of pulmonary tumor emboli after TAE. Cardiovasc Intervent Radiol. 1997. 20:139–141.

26. Wellons E, Rosenthal D, Schoborg T, Shuler F, Levitt A. Renal cell carcinoma invading the inferior vena cava: use of a "temporary" vena cava filter to prevent tumor emboli during nephrectomy. Urology. 2004. 63:380–382.

27. Blute ML, Boorjian SA, Leibovich BC, Lohse CM, Frank I, Karnes RJ. Results of inferior vena caval interruption by greenfield filter, ligation or resection during radical nephrectomy and tumor thrombectomy. J Urol. 2007. 178:440–445.

28. Sosa RE, Muecke EC, Vaughan ED Jr, McCarron JP Jr. Renal cell carcinoma extending into the inferior vena cava: the prognostic significance of the level of vena caval involvement. J Urol. 1984. 132:1097–1100.

29. Hedderich GS, O'Connor RJ, Reid EC, Mulder DS. Caval tumor thrombus complicating renal cell carcinoma: a surgical challenge. Surgery. 1987. 102:614–621.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download