Abstract
The incidence of specific intracranial parenchymal lesions of HIV-infected patients varies considerably between countries. In the Republic of Korea, the number of HIV-infected patients is increasing, but little is known regarding the spectrum of intracranial parenchymal lesions in these patients. The aim of the present study was to obtain this information. To identify HIV patients with intracranial parenchymal lesions, the electronic database of radiological reports for 1,167 HIV-infected patients, seen from 1999 to 2008 at the Seoul National University Hospital, were reviewed. Neuroradiologic studies were performed on 165 of these patients, and intracranial parenchymal lesions were detected in 40 (3.4%) of them. Thirty-seven were male, and median age was 41 yr (range, 26-61). At the time of the diagnosis of intracranial parenchymal lesions, median CD4+ lymphocyte count was 40 cells/µL (range 5-560) and in 33 (82.5%) patients, it was less than 200 cells/µL. Progressive multifocal leukoencephalopathy (12 patients) is the most frequent intracranial parenchymal lesions, followed by intracranial tuberculoma (7 patients), primary central nervous system lymphoma (7 patients), intracranial cryptococcoma (4 patients), Toxoplasma encephalitis (4 patients), and disseminated non-tuberculous mycobacterial infection (3 patients).
Neurologic manifestations are frequent in patients with human immunodeficiency virus (HIV) infection. They constitute the initial presentation in 10% of patients, and 30% to 50% develop neurologic complications during the course of the disease (1). Autopsy shows involvement of the nervous system in up to 80% of cases (2). Patients with HIV infection presenting with changed mental status or abnormal neurologic examination are frequently found to have intracranial parenchymal lesions (3). Various HIV related opportunistic infections and malignancies can cause these lesions (4). The relative frequencies of specific intracranial parenchymal lesions are very variable between countries, since the mode of infection and prevalence of infection with particular microorganisms such as Toxoplasma gondii and Epstein Barr virus (EBV) differ (5).
Since the first case of acquired immunodeficiency syndrome (AIDS) in Korea reported in 1985, the number of HIV-infected patients has increased every year (6). As of December 2008, the cumulative number of documented patients with HIV infection in Korea was 6,120 (7). However, although Kim et al. (8) reported neurologic complications of 34 Korean patients with HIV infection, knowledge of the spectrum of intracranial parenchymal lesions is limited to a few case reports (9-11). Hence, we have investigated the spectrum of intracranial parenchymal lesions in HIV-infected patients in the Republic of Korea.
We reviewed the medical records of 1,167 HIV-infected patients who were at least 16 yr old and visited the Seoul National University Hospital Infectious Disease Clinic more than once between January 1999 and December 2008. The patients with intracranial parenchymal lesions were identified from the electronic database of radiological reports. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-0910-018-296).
Diagnoses of intracranial parenchymal lesions were classified as definite, presumptive, or probable. A definite diagnosis was defined as a diagnosis based on histological proof of a cerebral disorder from a brain tissue specimen obtained by stereotactic brain biopsy. Presumptive diagnostic criteria for intracranial parenchymal lesions were as follows: 1) presumptive progressive multifocal leukoencephalopathy (PML), defined as positive JC virus DNA on polymerase chain reaction (PCR) from a cerebrospinal fluid (CSF) specimen, accompanied by a typical magnetic resonance imaging (MRI) pattern (12); 2) presumptive intracranial tuberculoma or disseminated non-tuberculous mycobacteria (NTM) disease, defined as evidence of lesions with mass effect from brain imaging and microbiological confirmation of tuberculosis or NTM from CSF or extra-cerebral specimens (13); 3) presumptive Toxoplasma encephalitis, defined as recent onset of focal neurologic abnormalities consistent with intracranial disease or a reduced level of consciousness, evidence of a lesion with mass effect or contrast enhancement by brain imaging, and positive serum antibody to T. gondii (14); 4) presumptive intracranial cryptococcoma, defined as evidence of lesions with mass effect from brain imaging and microbiological confirmation of Cryptococcus neoformans from CSF specimens (15); 5) presumptive cytomegalovirus encephalitis, defined as compatible neuroradiologic findings and positive cytomegalovirus DNA on PCR from CSF specimen (16). Probable diagnoses were determined by a board-certified infection disease specialist based on clinical and neuroradiological findings; the criteria were as follows (17): 1) probable PML, defined as presence of steady progression of focal neurologic deficit with a typical MRI pattern, namely asymmetric, non-enhancing, T2-hyperintense white matter abnormalities without mass effect; 2) probable HIV encephalopathy, defined as presence of progressive cognitive impairment with diffuse, bilateral, non-enhancing white-matter hyperintense lesions on MRI, and exclusion of opportunistic central nervous system (CNS) infections by examination of CSF; 3) probable primary CNS lymphoma, defined as evidence of a lesion with mass effect from brain imaging, exclusion of opportunistic CNS infections by examination of CSF, and response to radiation and/or chemotherapy.
Continuous variables are expressed as median and interquartile range (IQR) values. Difference in the incidence of intracranial parenchymal lesions by calendar years were analyzed using the Poisson regression. Statistical analyses were performed with SPSS software (version 17.0).
We treated 1,167 HIV-infected patients between 1999 and 2008. Total follow-up duration was 4,304 person-years and median follow-up duration per person was 3.16 yr (IQR, 0.93-6.03).
One thousand and sixty seven (91.4%) of the patients were male, 442 (41.4%) of whom had the risk factor of homosexual behavior. All patients were Korean and the median age was 37 yr (IQR, 30-45). The median CD4+ lymphocyte count at first visit to the study hospital was 230 cells/µL (IQR, 96-360), and the median CD4+ lymphocyte count at nadir during follow-up was 200 cells/µL (IQR, 80-300). Of 938 patients tested for antibody against T. gondii, 41 (4.3%) were positive (Table 1).
We performed neuroradiologic studies on the 165 patients with neurological manifestation reported among the 1,167 eligible patients, and found intracranial parenchymal lesions in 40 (3.4%) of them. Thirty three (82.5%) of the intracranial parenychymal lesions were multiple lesion, and 23 (57.5%) were enhanced on contrast-enhanced MRI. The overall incidence of intracranial parenchymal lesions was 0.93 per 100 person-years, and the incidence of intracranial parenchymal lesions (per 100 person-years) declined from 2.21 in 1999 to 0.69 in 2008 (P=0.003).
Of the 40 patients with intracranial parenchymal lesions, 37 (92.5%) were male, and the median age was 41 yr (IQR, 35-48). For 19 (47.5%) of these patients, the intracranial parenchymal lesion was the initial presentation of HIV infection. The median CD4+ lymphocyte count at nadir during follow-up was 28 cells/µL (IQR, 10-95). At the time of the diagnosis of the intracranial parenchymal lesions, median CD4+ lymphocyte count was 40 cells/µL (IQR, 13-150) and in 33 (82.5%) of the patients, the CD4+ lymphocyte count was less than 200 cells/µL (Fig. 1).
A definite diagnosis was obtained in 12 (30%) of the 40 patients with intracranial parenchymal lesions, a presumptive diagnosis was obtained in 19 (47.5%), and a probable diagnosis was obta-ined in nine (22.5%). All of the diagnoses were included among AIDS-defining diseases. PML was diagnosed in twelve cases (30.0%), intracranial tuberculoma in seven (17.5%), primary CNS lymphoma in seven (17.5%), cerebral cryptococcoma in four (10.0%), Toxoplasma encephalitis in four (10.0%), disseminated NTM disease in three (7.5%), HIV encephalopathy in two (5.0%) patients, and CMV encephalitis in one (Table 2).
Of the 12 patients with PML, one (8.3%) died during the observation period, two (16.7%) were lost to follow-up, and nine (75.0%) were improved with antiretroviral therapy. Of the 7 patients with intracranial tuberculoma, one (14.3%) died during the observation period, and six (85.7%) were improved with anti-mycobacterial treatment. Of the 7 patients with primary CNS lymphoma, two (28.6%) died during the observation period, one (14.3%) was lost to follow-up, and four (57.1%) were improved with systemic chemotherapy and/or cranial radiation therapy. The clinical features of the patients are summarized in Table 3.
In the present study, PML, intracranial tuberculoma, and primary CNS lymphoma were the most frequent intracranial parenchymal lesions. This situation contrasts with the outcome in other countries where Toxoplasma encephalitis is reported to be the most frequent intracranial parenchymal lesions (2-5).
Toxoplasma encephalitis is the most common intracranial parenchymal lesion in patients with AIDS in most Western and African countries. In the United States, where the prevalence of seropositivity for T. gondii in adult population is around 30%, Toxoplasma encephalitis develops in 3% to 10% of patients with AIDS (18). In contrast, the seroprevalence of T. gondii ranges from 1% to 10% in the Korean population (19, 20), and it was 4% in the present study. This might be the reason for low prevalence of Toxoplasma encephalitis in Korea (21). Our results may also have been affected by the introduction of prophylaxis and highly active antiretroviral therapy (HAART). In the United State, the incidence of Toxoplasma encephalitis decreased initially due to widespread prophylaxis for pneumocystis pneumonia using trimethoprim-sulfamethoxazole, which also prevents toxoplasmosis (22). Since the era of HAART, there has been a further decline in the incidence of Toxoplasma encephalitis (23). As the HAART has been available in Korea since 1999, it may have started to influence the incidence of Toxoplasma encephalitis.
PML is caused by the polyomavirus JCV. This double-stranded DNA virus infects 90% of the normal adult population worldwide and remains quiescent in the kidney without causing any disease. In conditions of immunosuppression, JCV is activated and undergoes lytic infection in oligodendrocytes, causing PML (24). Before the AIDS era, PML was a rare disease affecting mainly patients with chronic lymphocytic leukemia, non-Hodgkin's lymphoma, or organ transplant recipients. At the beginning of the AIDS epidemic, up to 5% of HIV-infected patients developed PML (25). Unlike the other opportunistic infections, the incidence of PML has not decreased since the availability of HAART and has even shown a slight increase; hence the importance of PML as a cause of intracranial parenchymal lesions is increasing (23). The introduction of HAART did not decrease the incidence of PML, but it improved the prognosis of PML. Before HAART era, the median interval from the time of symptom presentation to death was 2 to 4 months (25). In the HAART era, median survival has increased to 10.5 months, and half the patients survive for more 1 yr (26). In the present study, 9 (75.0%) of the 12 patients with PML improved with antiretroviral therapy, two were lost to follow-up, and only one died.
Intracranial tuberculoma is one of the most important causes of intracranial parenchymal lesions among AIDS patients, and is found in 10% of patients co-infected with HIV and tuberculosis (27). Because tuberculosis is the most frequent HIV-related opportunistic infection in HIV-infected patients in Korea (28, 29), we expected that intracranial tuberculomas would also be common in our population. During the study period, 73 patients with HIV infection were admitted for tuberculosis. Seven (9.6%) of them had intracranial tuberculomas.
Primary CNS lymphoma, which affected 2% of patients with AIDS at the beginning of the epidemic, has seen its incidence decrease considerably in the HAART era (30). In the present study, it developed in five patients; none of whom received antiretroviral therapy at the time of diagnosis. Detection of EBV DNA by PCR in the CSF is reported to have a sensitivity of 80% to 90% and a specificity of 87% to 98% for the diagnosis of primary CNS lymphoma (31), but we did not test any patients for EBV DNA of CSF in the present study.
Cryptococcus neoformans infection in patients infected with HIV usually causes subacute meningitis, but on rare occasion, it causes intracranial parenchymal lesions such as cryptococcoma (15). In some patients with cryptococcal meningitis, the use of HAART can cause immune reconstitution inflammatory syndrome (IRIS) (32). IRIS associated with cryptococcal infection usually presents as culture-negative meningitis, but sometimes as cryptococcoma (32). In the present study, 10 patients were admitted for cryptococcal meningitis; two had intracranial parenchymal lesions when initially diagnosed with cryptococcal meningitis, and two had intracranial cryptococcoma with IRIS.
There are some limitations to the present study. First, the incidence of intracranial parenchymal lesions might be underestimated, because some patients with intracranial parenchymal lesions may not have been evaluated despite the presence of neurologic problems. Second, since definite and presumptive diagnoses were achieved in only 77% of the patients, the present study results may not reflect the true spectrum of intracranial parenchymal lesions entirely accurately. However, the data obtained are significant as representing the first description of the spectrum of intracranial parenchymal lesions in HIV-infected patients in Korea.
In the present study, we investigated 1,167 HIV patients, making up 19.1% of all Korean patients with HIV infection as of December 2008, and there were no significant differences between the demographic findings including age, sex, and transmission route for the present study population and the national statistics for all Korean patients with HIV infection (7). Among the intracranial parenchymal lesions developed in Korean patients with HIV infection in the HAART era, PML is the most frequent cause, followed by intracranial tuberculoma, primary CNS lymphoma, intracranial cryptococcoma, and Toxoplasma encephalitis.
Figures and Tables
Fig. 1
CD4+ lymphocyte counts at the time of diagnosis of each intracranial parenchymal lesion in HIV-infected patients. Bars denote median values.
PML, Progressive multifocal leukoencephalopathy; PCNSL, Primary central nervous system lymphoma; TOXO, Toxoplasma encephalitis; NTM, Disseminated non-tuberculous mycobacteria disease; CMV, Cytomegalovirus encephalitis.

Table 1
Baseline characteristics of 1,167 study patients and HIV-infected patients with intracranial parenchymal lesions seen at Seoul National University Hospital, January 1999 to December 2008
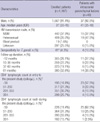
Table 2
Prevalence of intracranial parenchymal lesions in 1,167 HIV-infected patients seen at Seoul National University Hospital, January 1999 to December 2008

Table 3
Clinical features and diagnoses in the HIV-infected patients with intracranial parenchymal lesions, seen at Seoul National University Hospital from 1999 to 2008

*CD4+ lymphocyte count and HIV RNA at the time of diagnosis of intracranial parenchymal lesions.
HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; SI, signal intensity; PML, progressive multifocal leukoencephalopathy; IRIS, immune reconstitutional inflammatory syndrome; DLBL, diffuse large B cell lymphoma; CSF, cerebrospinal fluid; PCR, polymerase chain reaction; CNS, central nervous system; N/V, nausea/vomiting; F/U, follow-up; NTM, non-tuberculous mycobacteria; Toxo, Toxoplasma gondii.
References
1. Levy RM, Bredesen DE, Rosenblum ML. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): experience at UCSF and review of the literature. J Neurosurg. 1985. 62:475–495.

2. De Girolami U, Smith TW, Henin D, Hauw JJ. Neuropathology of the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1990. 114:643–655.
3. Jellinger KA, Setinek U, Drlicek M, Bohm G, Steurer A, Lintner F. Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol. 2000. 100:213–220.

4. Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000. 14:69–74.

5. Davies J, Everall IP, Weich S, Glass J, Sharer LR, Cho ES, Bell JE, Majteny C, Gray F, Scaravilli F, Lantos PL. HIV-associated brain pathology: a comparative international study. Neuropathol Appl Neurobiol. 1998. 24:118–124.

6. Oh MD, Choe K. Epidemiology of HIV infection in the Republic of Korea. J Korean Med Sci. 1999. 14:469–474.

7. KCDC Public Health Weakly Report. KCDC. accessed on 1 September 2009. Available at http://www.cdc.go.kr/phwr.
8. Kim HJ, Kim S, Lee KB, Lee KW, Oh MD, Choe KW. Neurologic complications of human immunodeficiency virus-type 1 infection. J Korean Med Sci. 2003. 18:149–157.

9. Lee EB, You KH, Oh MD, Kim NJ, You CD, Baek HJ, Shin HS, Heo DS, Chi JG, Choe KW. A case of primary central nervous system lymphoma with acquired immunodeficiency syndrome. Korean J Infect Dis. 1996. 28:367–372.
10. Lee KD, Park WB, Jung HS, Kang CI, Kim DM, Kim HB, Oh MD, Choe KW. Mycobacterium avium-intracellularae meningoencephalitis in a patient with acquired immunodeficiency syndrome. Infect Chemother. 2003. 35:306–309.
11. Kim BH, Lee SI, Lee CH, Cha SH, Lee TH, Lee SH, Chung JS, Cho GJ. A case of cerebral toxoplasmosis in a patient with acquired immune defeciency syndrome. Infect Chemother. 2004. 36:181–184.
12. Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999. 52:253–260.

13. Thonell L, Pendle S, Sacks L. Clinical and radiological features of South African patients with tuberculomas of the brain. Clin Infect Dis. 2000. 31:619–620.

14. Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992. 41:1–19.
15. Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, Sobel JD, Dismukes WE. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000. 30:710–718.
16. Cinque P, Vago L, Brytting M, Castagna A, Accordini A, Sundqvist VA, Zanchetta N, Monforte AD, Wahren B, Lazzarin A. Cytomegalovirus infection of the central nervous system in patients with AIDS: diagnosis by DNA amplification from cerebrospinal fluid. J Infect Dis. 1992. 166:1408–1411.

17. Portegies P, Solod L, Cinque P, Chaudhuri A, Begovac J, Everall I, Weber T, Bojar M, Martinez-Martin P, Kennedy PG. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004. 11:297–304.

18. Luft BJ, Hafner R, Korzun AH, Leport C, Antoniskis D, Bosler EM, Bourland DD 3rd, Uttamchandani R, Fuhrer J, Jacobson J, Morlat P, Vlide JL, Remington JS. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1993. 329:995–1000.

19. Song KJ, Shin JC, Shin HJ, Nam HW. Seroprevalence of toxoplasmosis in Korean pregnant women. Korean J Parasitol. 2005. 43:69–71.

20. Shin DW, Cha DY, Hua QJ, Cha GH, Lee YH. Seroprevalence of Toxoplasma gondii infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J Parasitol. 2009. 47:125–130.

21. Lee SH, Lee SH, Cha DH, Lee SJ, Kwak IS, Chung JS, Cho GJ, Lee H, Jung DS, Moon CS, Park JY, Ko OB, Shin KD. Clinical characteristics and prevalence of toxoplasma infection in human immunodeficiency virus-infected patients in South Korea. Korean J Med. 2009. 76:713–721.
22. Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009. 58:1–207.

23. Ammassari A, Cingolani A, Pezzotti P, De Luca DA, Murri R, Giancola ML, Larocca LM, Antinori A. AIDS-related focal brain lesions in the era of highly active antiretroviral therapy. Neurology. 2000. 55:1194–1200.

24. Koralnik IJ. New insights into progressive multifocal leukoencephalopathy. Curr Opin Neurol. 2004. 17:365–370.

25. Berger JR, Kaszovitz B, Post MJ, Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med. 1987. 107:78–87.
26. Tantisiriwat W, Tebas P, Clifford DB, Powderly WG, Fichtenbaum CJ. Progressive multifocal leukoencephalopathy in patients with AIDS receiving highly active antiretroviral therapy. Clin Infect Dis. 1999. 28:1152–1154.
27. Bishburg E, Sunderam G, Reichman LB, Kapila R. Central nervous system tuberculosis with the acquired immunodeficiency syndrome and its related complex. Ann Intern Med. 1986. 105:210–213.

28. Oh MD, Park SW, Kim HB, Kim US, Kim NJ, Choi HJ, Shin DH, Lee JS, Choe K. Spectrum of opportunistic infections and malignancies in patients with human immunodeficiency virus infection in South Korea. Clin Infect Dis. 1999. 29:1524–1528.

29. Kim JM, Cho GJ, Hong SK, Chang KH, Chung JS, Choi YH, Song YG, Huh A, Yeom JS, Lee KS, Choi JY. Epidemiology and clinical features of HIV infection/AIDS in Korea. Yonsei Med J. 2003. 44:363–370.

30. Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002. 8:Suppl 2. 115–121.

31. Bossolasco S, Cinque P, Ponzoni M, Vigano MG, Lazzarin A, Linde A, Falk KI. Epstein-Barr virus DNA load in cerebrospinal fluid and plasma of patients with AIDS-related lymphoma. J Neurovirol. 2002. 8:432–438.

32. Shelburne SA 3rd, Darcourt J, White AC Jr, Greenberg SB, Hamill RJ, Atmar RL, Visnegarwala F. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005. 40:1049–1052.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download