Abstract
The study of cancer in patients treated with dialysis in Korea has not been reported. The aim of this study was to investigate the incidence and mortality of cancer among patients on dialysis in Korea. The study subjects were 106 cancer patients (2.3%) out of 4,562 end-stage renal disease (ESRD) patients maintained on hemodialysis (HD) or peritoneal dialysis (PD) at Yonsei University Health System from 1996 to 2005. We excluded patients in whom the diagnosis of cancer preceded dialysis or those who received renal allograft or started dialysis after renal allograft. Seventy-three (69%) of our subjects were male and 33 (31%) were female. The mean age at the time of cancer diagnosis was 57.9±11.7 yr. The mean time from the start of dialysis to the diagnosis of cancer was 75.2±63.9 months. The most common cancer site was gastrointestinal tract (GIT) (51%) followed by urinary tract (20%), lung (8%), and thyroid (7%). Sixty nine percent of the total mortality was due to cancer. The mean time from diagnosis to death was 2.9±2.5 yr. In ESRD patients with cancer, there were no significant differences in mortality rates by dialysis modality. In ESRD patients, the most common cancer was GIT cancer followed by urinary tract cancer. Therefore, careful surveillance of these cancers in ESRD patients is highly recommended.
Malignant tumors are the leading cause of death in the general population worldwide. Although cardiovascular disease is the most common cause of death in patients with chronic kidney disease (CKD) (1, 2), the incidence of malignant diseases is growing in this population due to increased life span. Many studies have reported that patients who received kidney transplant are at higher risk of malignant disease (3-6). However, whether CKD patients undergoing dialysis treatment have similar risks of developing malignancies is still controversial (6-10), although most studies have shown that this risk of developing malignant disease in CKD patients (with or without dialysis) is increased in comparison with the general population (3-13%) (3, 7, 11). The exact mechanisms responsible for this increased incidence of cancer have not yet been fully elucidated but several factors such as impaired immune system, chronic infections, decreased antioxidant capacity, accumulation of carcinogenic compounds, and dialysis-related factors may promote malignant transformation and tumor formation (12-16).
As studies of cancer in CKD patients on dialysis in Korea have not yet been reported, we undertook this study to investigate the clinical features and outcomes of cancer in Korean dialysis patients.
We conducted a retrospective study of a total of 4,562 endstage renal disease (ESRD) patients who started hemodialysis (HD) or peritoneal dialysis (PD) at two centers at Yonsei University (Shin-chon and Young-Dong Severance Hospital) from January 1996 to December 2005. Among the study population, malignant diseases were diagnosed in 106 patients (2.3%). Malignancies were detected by imaging methods such as computed tomography (CT) or magnetic resonance imaging (MRI) and by histological methods. Patients who developed malignancies prior to dialysis or who received kidney transplant were excluded. Medical records were reviewed and used to collect data such as gender, age, underlying renal diseases, and the interval from the initiation of dialysis to the development of a malignant tumor. The cause of death and age at the time of death of the ESRD patients with cancer were obtained from the Korea National Statistical Office.
For comparison with the general population, the medical records of 53,720 patients diagnosed with cancer during the same period at our hospital were also examined. This group did not include CKD patients (with or without dialysis).
All data were expressed as mean±SD. Statistical analysis was performed using Student's t-test and the chi-square test, and a value of p<0.05 was considered to indicate significant difference. The significance of differences in survival rates between HD and PD patients was determined using the Kaplan-Meier method. The standardized incidence rate (SIR: the ratio of observed to expected cancers) was used to estimate the relative risk and 95% confidence interval (CI) for these ratios were calculated with the assumption that the observed number of cancer cases followed a Poisson distribution. Data analysis was done with SAS software (version 6.12).
Of the total of 66,025 patients with malignancies registered from January 1 to December 31, 2002, 55,398 (55.9%) cases were male and 43,627 (44.1%) cases were female: a sex ratio of 1.27:1. The leading age groups in order of relative frequency were 60-64 yr of age (14.8%), followed by 65-69 (14.4%), 55-59 (11.7%), 70-74 (10.7%), 50-54 (9.9%), and 45-49 (9.0%). The relative frequencies of age groups below 45 and above 75 were 0.1-7.5% and 10.9%, respectively. The leading primary cancer sites in order of relative frequency were stomach (20.2%), followed by bronchus and lung (11.9%), liver and intrahepatic bile ducts (11.3%), colorectum (11.2%), breast (7.4%), thyroid (4.9%), uterine cervix (4.0%), hematopoietic systems (2.6%), pancreas (2.4%), and urinary bladder (2.2%).
In males, the leading primary cancer sites were stomach (24.0%), followed by bronchus and lung (15.0%), liver and intrahepatic bile ducts (15.4%), colorectum (11.6%), urinary bladder (3.2%), prostate (3.0%), esophagus (2.8%), hematopoietic systems (2.7%), pancreas (2.5%), and kidney (2.0%). In females, breast (16.8%) was the most common site, followed by stomach (15.3%), colorectum (10.7%), thyroid (9.5%), uterine cervix (9.1%), bronchus and lung (6.6%), liver and intrahepatic bile ducts (6.0%), ovary (3.6%), hematopoietic systems (2.5%), and pancreas (2.3%).
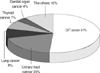
The mean age of the general population at the time of the diagnosis of a malignant tumor was 53.0±18.1 yr (male:female=28,335:25,385). The most common cancer was gastrointestinal tract (GIT) cancer (44%), followed by genital organ cancer (18%), lung cancer (9%), thyroid cancer (7%), and urinary tract cancer (4%). The remaining cancers were head and neck cancer (4%), hematologic cancer (3.3%), skin cancer (1%), and cancer of unknown origin (Fig. 1).
Among the 4,562 ESRD patients, 106 (2.3%) were diagnosed with malignant tumors. Seventy three patients were male (69%) and 33 were female (31%) (2.2:1). Unlike in the general population, malignancies developed predominantly in the males of our study population (p<0.05). Diabetic nephropathy (42%), hypertensive nephropathy (18%), and chronic glomerulonephritis (16%) were the most common causes of ESRD. Among the 106 patients, 63 (59%) received HD, and 43 received PD (41%). The mean age at the time of diagnosis of the cancer was 57.9±11.7 yr (26-83 yr), higher than the general population (57.9±11.7 vs. 53.0±18.1 yr, p<0.05). The mean duration from the initiation of dialysis to diagnosis was 75.2±63.9 months (3-288 months) (Table 1). Follow-up duration from diagnosis was 42.9±47.3 months (1-240).
In the general population, common cancer sites were stomach (22.0%), liver (16.3%), lung (12.3%), and intestine (10.8 %) in males, whereas in females, the incidence of female genital tract cancer, including uterine cervical cancer (19.4%) and breast cancer (17.9%), was the highest, followed by stomach (12.0%), thyroid (13.2%), and intestine (7.7%). On the other hand, in ESRD patients, liver was the most common cancer site followed by intestine and stomach in male patients. In female patients, urinary tract cancer showed the highest incidence rate (Table 3).
The earliest diagnosed cancer was multiple myeloma and the latest was cancer of liver (mainly hepatocellular carcinoma, HCC). For cancer of liver, kidney and thyroid, the interval to diagnosis of malignant tumors was relatively long (Table 4). Among the 106 patients, 18 (17%) were diagnosed with malignant tumors within 1 yr of beginning dialysis, and the remaining 88 (83%) were diagnosed within 10 yr (Table 5). The risk of cancer was slightly higher when cancer was diagnosed during the first year of dialysis.
When ESRD patients were divided into two groups according to age of 40, most malignancies developed after 40 yr of age (84%); Malignancies of patients older than 40 yr (n=89) includes 47 GIT cancers, 14 urinary tract cancer, 9 lung cancers, 4 thyroid cancers, and 15 other cancers. In patients less than 40 yr of age (n=18), there were 7 GIT cancer, 7 urinary tract cancer, and 4 thyroid cancers (Fig. 3A). In the general patients older than 40 yr, the leading primary cancer sites were GIT (45%), followed by breast & uterine cervix (19%), lung (9%), and thyroid (7%). In the general patients younger than 40 yr, breast & uterine cervix (30%) was the most common site, followed by GIT (22%), thyroid (15%), and hematologic systems (5%) (Fig. 3B).
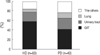
Fig. 4 shows the cancer distribution with respect to dialysis modality. We found no significant difference between HD and PD treated patients. In HD (n=63) patients, GIT cancer was the most common (n=37, 58.7%), followed by urinary tract cancer (n=12, 19%) and lung cancer (n=4, 6.3%), with a similar pattern seen in PD (n=43) patients.
The mean time from diagnosis to death was 2.9±2.5 yr. Among the 106 cancer patients, the overall mortality rate was 53%. Thirty four patients (60%) died of cancer and 22 (40%) died of cause not related to cancer, such as cerebral hemorrhages (n=3), cardiovascular disease (n=3), sepsis (n=2), trauma (n=1) and unknown reasons (n=13). Deaths occurred in all patients with cancer of biliary tract system, in 2 out of 3 patients with hematologic cancer (67%), in 5 out of 9 patients with lung cancer (56%), and in 6 out of 13 patients with bladder cancer (46%) (Table 6).
The survival rate of ESRD patients without cancer was significantly higher compared to that of ESRD patients with cancer (p<0.05). In ESRD patients with cancer, there were no significant differences in mortality rates by dialysis modality (Fig. 5). Five year survival rate was 44.0% and 46.6% in patients treated with HD and PD respectively.
CKD patients have a high incidence of cancer for several reasons, including: weakened immune system, previous immunosuppressive treatment, altered DNA repair, and chronic infection (12-16).
In 25,044 patients who received dialysis in the U.S.A., Europe, Australia, or New Zealand, the overall risk of cancer is increased (3%) compared with the general population (17). In one report, HD patients have a greater risk of developing malignant tumors than the general population, with malignancies diagnosed in 4.9% of patients (18). In a recent meta-analysis of 15 studies, it was found that the relative risk (observed cancer cases over expected cancer cases) averaged 7.6 in 10 studies (72,484 ESRD patients). In contrast, in the remaining 5 studies (35,407 ESRD patients) the average risk was 0.98 (19). Despite this lack of consensus, there is general agreement that CKD patients have a greater risk of cancer than the general population. A recent report showed that cancer incidence is increasing in Korea, with an incidence rate of 242.3 per 100,000 (0.24%) for men from 1998 to 2002 (20). In our study, 106 (2.3%) organ tumors were found in 4,562 CKD patients, which is a higher incidence of cancer compared with the general population, consistent with previous reports. However, we also found the incidence of malignancies in ESRD patients to be lower than in previous reports (3-13%) (3, 7, 11). Although we have no clear explanation for this discrepancy, it may partly be explained by our research having been conducted more recently and, possibly, differences in institution size and race.
In 2002 annual report of the Korea central cancer registry, the leading cancer site was stomach followed by lung and liver in males whereas it was breast followed by stomach, colorectum, and thyroid in females. On the other hand, in ESRD patients, the most common cancer sites were liver, colorectum, and urinary tract in males and urinary tract, thyroid, and lung in female in descending order.
Several previous studies (7, 21) have reported that urologic malignancies, which are not that common in the general population, are the most common cancer in chronic hemodialysis patients as well as nondialysed uremic patients. Cengiz et al. (22) reported that 19% of CKD patients (regardless of dialysis treatment) had a urologic malignancy. In other reports, bladder cancer developed in 28.5% of HD patients (23) and kidney and bladder cancers (12 of 33) were the most common types of cancer in HD patients (5). Ishikawa et al. (24) also showed that the incidence of renal cell carcinoma in HD patients was 41 times that of the general population. In contrast, our study found GIT cancers to be the most common in ESRD patients, although urologic malignancy were more prevalent than in the general population, which is similar to the findings of a Japanese survey (25). In fact, these results are not surprising, as stomach cancer is the most frequent in Japan and Korea.
The mean age at the time cancer diagnosis in ESRD patients was higher than in the general population. This may be because the age of dialysis patients is relatively higher than the general population as a whole, thus causing the age at the time of cancer diagnosis to also be high. According to the incidence rate by the dialysis duration, 17% of all malignancies occurred within 1 yr of starting dialysis, with a gradually decreasing trend in the malignancy incidence rate afterwards. This finding was in line with a report by Inamoto et al., reporting that the incidence was very high during the first 6 months of dialysis treatment and rather low in the 7th-10th year. (25). However, compared to the study by Inamoto et al, only 17% of all malignancies occurred within 1 yr of starting dialysis in our study. This is partly because that our study include many cases of HCC, thyroid cancer, etc., which generally have a relatively delayed development, and that could explain why we found relatively few malignancies within 1 yr of beginning dialysis. The risk of cancer was slightly higher when cancer was diagnosed during the first year of dialysis. This finding might be due to increased detection of cancer and more detailed observation at the time of initial dialysis treatment.
Similar to the general population, GIT cancers were the most common in our subjects, implying that early detection and aggressive treatment of these cancers is just as important in ESRD patients as in the general population. In addition, the incidence of urinary tract cancer was substantially higher than in the general population, indicating that it is important to perform screening tests for this type of cancer as well. Unlike in transplant recipients (26-28), the types of cancers most commonly diagnosed in patients on dialysis are those most likely to be found in the general non-ESRD population. These results should reinforce the need for routine check-up to include renal and gastrointestinal tract cancer when dialysis patients develop even minor symptoms. Increased surveillance should lead to detection of cancers at an earlier stage, which are not observed among GIT and urinary tract cancers.
Our findings showed that liver cancers were more prevalent in ESRD patients. Infection with HBV or HCV is a serious problem in dialysis patients. The fact that ESRD patients have greater exposure to hepatitis B and C viruses than normal patients, probably accounts for the observed excess of liver cancer (29). Therefore, widespread screening for HBV and HCV also should be encouraged.
It should be noted that all 3 hematologic malignancies which occurred in ESRD patients were multiple myeloma. Multiple myeloma (MM) is a common hematologic malignancy and also can be the cause of ESRD (30). Consequently, the diagnosis of MM might be missed at the time of the dialysis start and it is possible that its incidence could be underestimated.
Interestingly, in our study, thyroid cancer was fairly common, it was found in 8 (7.5%) patients. Thyroid disease, including hypothyroidism, goiter, nodules, and thyroid cancer, may occur more frequently in ESRD patients than in the general population (17, 31), and it has been suggested that chronic immunosuppression and secondary hyperparathyroidism may play a role (32, 33).
There are several limitations to the present study. First, we analyzed limited data from two centers in a retrospective review. Second, we had more limited follow-up and fewer observed cancer cases than those might be found in a larger cohort of dialysis patients. Third, the relationship between the underlying disease and malignancy was not evaluated. However, even with such limitations, this study contributes to a comprehensive assessment of cancer risk in ESRD patients, especially in Korea.
In conclusion, our data shows that ESRD patients in Korea have a higher risk of developing cancer than the general population, and the types of cancer most commonly diagnosed in ESRD patients are those most likely to be found in the general population. In addition, the incidence of urinary tract cancer is higher in ESRD patients compared to the general population, thus, careful surveillance for malignancies in ESRD patients, especially for those of the urinary tract, is highly recommended.
Figures and Tables
Fig. 3
Frequency of cancer by age between ESRD patients and general population. (A) ESRD patients, (B) general population.

Fig. 5
Survival of ESRD patients with cancer. (A) Comparison of survival rate between ESRD patients without cancer and ESRD patients with cancer, (B) Comparison of survival rate by dialysis modality in ESRD patients with cancer.

References
1. Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000. 58:353–362.

2. Lameire N, Bernaert P, Lambert MC, Vijt D. Cardiovascular risk factors and their management in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 1994. 48:Suppl. S31–S38.
3. Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1994. 99–109.
4. Brunner FP, Landais P, Selwood NH. Malignancies after renal transplantation: the EDTA-ERA registry experience: European Dialysis and Transplantation Association-European Renal Association. Nephrol Dial Transplant. 1995. 10:Suppl 1. 74–80.
5. Pecqueux JC, Schwarz A, Dieckmann KP, Offermann G. Cancer incidence in patients on chronic dialysis and in renal transplant recipients. Urol Int. 1990. 45:290–292.

6. Sheil AG, Flavel S, Disney AP, Mathew TH. Cancer development in patients progressing to dialysis and renal transplantation. Transplant Proc. 1985. 17:1685–1688.
7. Matas AJ, Simmons RL, Kjellstrand CM, Buselmeier TJ, Najarian JS. Increased incidence of malignancy during chronic renal failure. Lancet. 1975. 1:883–886.

8. Kinlen LJ, Eastwood JB, Kerr DN, Moorhead JF, Oliver DO, Robinson BH, de Wardener HE, Wing AJ. Cancer in patients receiving dialysis. Br Med J. 1980. 280:1401–1403.

9. Ota K, Yamashita N, Suzuki T, Agishi T. Malignant tumors in dialysis patients:a nationwide survey. Proc Eur Dial Transplant Assoc. 1981. 18:724–730.
10. Bush A, Gabriel R. Cancer in uremic patients. Clin Nephrol. 1984. 22:77–81.
12. Schollmeyer P, Bozkurt F. The immune system of the uremic patient: hemodialysis vs CAPD. Clin Nephrol. 1988. 30:Suppl. S37–S40.
13. Bonomini M, Forster S, De Risio F, Rychly J, Nebe B, Manfrini V, Klinkmann H, Albertazzi A. Effects of selenium supplementation on immune parameters in chronic uraemic patients on haemodialysis. Nephrol Dial Transplant. 1995. 10:1654–1661.
14. Fabrizi F, Marcelli D, Bacchini G, Guarnori I, Erba G, Locatelli F. Antibodies to hepatitis C virus (HCV) in chronic renal failure (CRF) patients on conservative therapy: prevalence, risk factors and relationship to liver disease. Nephrol Dial Transplant. 1994. 9:780–784.
15. Yanagisawa H, Manabe S, Kanai Y, Wada O. Carcinogenic glutamic acid pyrolysis product in the dialysate of uremic patients treated by continuous ambulatory peritoneal dialysis. Clin Nephrol. 1988. 30:73–78.
16. Akizawa T, Kinugasa E, Koshikawa S. Increased risk of malignancy and blood-membrane interactions in uraemic patients. Nephrol Dial Transplant. 1994. 9:Suppl 2. S162–S164.
17. Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, McCredie M, Boyle P. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999. 354:93–99.

18. Cuckovic C, Djukanovic L, Jankovic S, Stanojcic A, Dragicevic P, Radmilovic A, Lambic L, Stojanovic M, Milic M, Bakovic J, Radovic M, Labudovic M. Malignant tumors in hemodialysis patients. Nephron. 1996. 73:710–712.

19. Marple JT, MacDougall M. Development of malignancy in the endstage renal disease patient. Semin Nephrol. 1993. 13:306–314.
20. Shin MH, Oh HK, Ahn YO. Ten year trend of cancer incidence in Seoul, Korea: 1993-2002. J Prev Med Public Health. 2008. 41:92–99.

21. Kjellstrand CM. Are malignancies increased in uraemia? Nephron. 1979. 23:159–161.
22. Cengiz K, Block AM, Hossfeld DK, Anthone R, Anthone S, Sandberg AA. Sister chromatid exchange and chromosome abnormalities in uremic patients. Cancer Genet Cytogenet. 1988. 36:55–67.

23. De Sala O'shea E, Morey Molina A, Ferrutxe Frau J, Gutierrez Sanz-Gadea C, Alarcon Zurita A, Ozonas Moragues M. Cancer of the bladder and hemodialysis. Arch Esp Urol. 1990. 43:359–363.
24. Ishikawa I. Renal cell carcinoma in chronic hemodialysis patients-a 1990 questionnaire study in Japan. Kidney Int. 1993. 41:Suppl. S167–S169.
25. Inamoto H, Ozaki R, Matsuzaki T, Wakui M, Saruta T, Osawa A. Incidence and mortality patterns of malignancy and factors affecting the risk of malignancy in dialysis patients. Nephron. 1991. 59:611–617.
26. Agraharkar ML, Cinclair RD, Kuo YF, Daller JA, Shahinian VB. Risk of malignancy with long-term immunosuppression in renal transplant recipients. Kindey Int. 2004. 66:383–389.

27. Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. 2004. 15:1582–1588.
28. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004. 4:905–913.

29. Niu MT, Coleman PJ, Alter MJ. Multicenter study of hepatitis C virus infection in chronic hemodialysis patients and hemodialysis center staff members. Am J Kidney Dis. 1993. 22:568–573.

30. Basi S, Schulman G, Fogo AB. Multiple complications in multiple myeloma. Am J Kidney Dis. 2005. 45:619–623.

31. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev. 1996. 17:45–63.

32. Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved. Am J Nephrol. 1998. 18:89–95.

33. Klyachkin ML, Sloan DA. Secondary hyperparathyroidism: evidence for an association with papillary thyroid cancer. Am Surg. 2001. 67:397–399.




 PDF
PDF Citation
Citation Print
Print









 XML Download
XML Download