Abstract
Urinary tract infections are common clinical problems in children, even though lots of treatment strategies have been tried. Many studies of the application of probiotics for urinary tract infection in female adults exist, but there is a lack of studies in children. The aims of this study were to screen probiotic strains for inhibiting the uropathogens in vitro, to find candidates for in vivo study. Nine strains of E. coli were isolated from children with urinary tract infection and six uropathogens were obtained from Korean Colletion for Type Cultures and American Type Culture Collection. Also 135 lactic acid bacteria (LAB) strains were isolated from healthy children, and were identified through physiologic, biochemical methods, 16S rDNA PCR, and data analysis. And with agar disk diffusion assay technique the antimicrobial activities of these LAB strains against those uropathogens were examined. Three strains of separated LAB strains demonstrated major antimicrobial activity against all the uropathogens. In the agar disk diffusion assay technique, antimicrobial activities increased most in the 4th day culture broth with separated Lactobacillus. In summary, some LAB can be used as candidates to develop the probiotic microorganisms that inhibit uropathogens in children, and are expected to be applied to treatment and prevention of pediatric urinary tract infection.
Urinary tract infections are common clinical entities occurring in a variety of pediatric patient groups. The causative organisms are known to include Escherichia coli, Proteus spp., Klebsiella spp., Staphylococcus spp., mainly intestinal uropathogens (1). Urinary tract infection is frequently accompanied with urologic abnormalities and can cause end stage renal failure or hypertension if continued. This results in using antibiotics for the purpose of controlling uropathogens, produces even superbacteria which are multi-resistant and cause intractable infections that are difficult to treat. So a lot of supplementary treatment strategies have been tried. It has been known for almost a century that beneficial urogenital normal flora play an important role in inhibition of uropathogens and prevent urinary tract infection (2). This concept of beneficial normal flora is probiotics. Probiotics are live microbial organisms that are administered in supplements to benefit the host (3, 4). Strains of Lactic acid bacteria (LAB), which comprise a clade of Gram positive, acid tolerant rod or cocci, are the most common microbes employed as probiotics.
Two principal kinds of probiotic bacteria are the genera Lactobacillus and Bifidobacterium (5). Applications of probiotics are widespread, with the primary applications being diarrhea (Lactobacillus rhamnosus GG) (6-8), allergic disorders (L. rhamnosus GG) (9), urinary tract infection and vaginitis of female adults (L. rhamnosus GR-1, L. fermentum B-54) (10).
There are many studies of the application of probiotics for urinary tract infection in female adults, but studies lack in children, especially in vivo studies. The aims of this study were to screen probiotic strains for inhibiting the uropathogenic E. coli and other uropathogens in vitro, to find candidates for in vivo study.
Forty eight E. coli strains were isolated from the urine of patients with urinary tract infection (>105 CFU/mL) from January 2006 to January 2007 in Department of Pediatrics, College of Medicine, Chung-Ang University Yongsan Hospital. The urine of patients was cultured on blood agar, Mac-Conkey agar and triple sugar iron agar plates and determined motility, indole, ornithine, citrate, lysine, decarboxylase for biochemical tests. Uropathogenic reference organisms were obtained from Korean Collection for Type Cultures (KCTC) and American Type Culture Collection (ATCC) (Table 1).
LAB were isolated from the feces of 50 children who visited Chung-Ang University Yongsan Hospital and were gastroenterologically healthy from October 2006 to January 2007. One gram of feces were taken for the isolation, diluted to 106-107 CFU/mL with sterilized phosphate buffered saline (PBS), smeared 0.1 mL on modified Man-Rogosa-Sharpe (MRS) media (Difco, Detroid, MI, U.S.A.), and cultured in 37℃ with 5% CO2 for 36 hr. LAB-like colonies were subcultured on blood-free egg yolk media (BCP), so that pure LAB colonies were obtained.
Identification of isolated LAB was performed by general features and biochemical tests using API 50 CHL Carbohydrate test kit (BioMerieux, Marcy l'Etoile, France). LAB maintained on modified MRS media were dissolved in the 5 mL solvent, adjusted for their number with Mcfarland Standard 2, and smeared on API 50 CHL media. Subsequently the media were filled with mineral oil, cultured in 37℃ for 48 hr, and confirmed by API Lab plus software (BioMerieux).
Genomic DNA was extracted using the cetyl trimethylammonium bromide (CTAB) method. The cultured LAB were taken to 1.5 mL tubes and centrifuged at 15,000 rpm for two minutes. The precipitated bacteria were dissolved with TE buffer (pH 8.0) 100 µL and reacted with 10% sodium dodecyl sulfate 20 µL and proteinase K (10 mg/mL) 10 µL at 50℃ for an hour. Ten percent CTAB/0.7M NaCl solution 200 µL was added, reacted at 65℃ for 10 min, subsequently the same amount of chloroform/isoamylalcohol (24:1) solution was added, shaken, and cultured for five minutes. Next, it was centrifuged at 4℃ for five minutes and the supernatants were moved to a new tube. To this tube, 1/10 times 3 M sodium acetate and twice 100% ethanol were added, so the genomic DNA was precipitated. The precipitates were washed with 70% ethanol, dissolved in TE buffer 50 µL, and RNA was removed by adding RNase and reacted at 42℃ for 30 min. The purified DNA was quantitated with spectrophotometer (Model MBA 2000, Perkin-Elmer, Norwalk, CT, U.S.A.) at 260 nm, and stored at -20℃.
The primers for amplification of 16S rDNA and sequencing analysis of LAB were produced based upon the report of Lane about E. coli 16S rDNA gene (11). The produced primers were diluted to 29 µM and to 1.6 pM for polymerase chain reaction and for sequencing, respectively, with sterilized distilled water, and stored at -20℃. 16S rDNA was amplified by adding 10× Taq buffer 10 µL, 2.5 mM dNTPs 8 µL, 1.25 mM MgCl2 10 µL, distilled water 59 µL, 10 µM 27f primer (5'-AGAGTTTGATCMTGGCTCAAG-3') 1 µL, 10 µM 1525r primer (5'-AAGGAGGTGWTCCARCC-3') 1 µL, and Taq polymerase (Roche, Basel, Switzerland) 0.5 µL to the extracted genomic DNA, 30 cycles of reactions at 94℃ for two minutes, at 55℃ for 1 min, and at 72℃ for 2 min in GeneAmp 2700 (Applied Biosystems, Foster City, CA, U.S.A.). Electrophoresis of amplified 16S rDNA in 1.0% LE agarose gel (FMC Bioproducts, Rockland, ME, U.S.A.) for 20 min was performed, and it was stained for 10 min with ethidium bromide (10 mg/mL), and checked the degree of amplification using Gel Doc 2000 system (Bio-Rad, Hercules, CA, U.S.A.).
Sequencing of the 16S rDNA was executed by using BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) and ABI PRISM 310 Genetic Analyzer (Applied Biosystems). Analysis of similarity was performed by searching BLAST server of National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast/) and using Clustal_X (version 1.7) program. Phylogenetic trees were produced with PHYLIP package and neighbor-joining algorithm, and evolutional distances were calculated with Jukes & Kantor neighbor-joining method.
From the identified LAB supernatants, antimicrobial activities against uropathogenic E. coli and other uropathogenic bacteria were checked using the agar disk diffusion assay technique. Uropathogenic organisms used in this study were nine strains of E. coli and six species of other pathogens from KCTC and American Type ATCC (Table 1). The culture fluids of uropathogens were inoculated into 10 mL nutrient media in 200 mL flasks, with initial O.D.=0.1, and cultured for seven hours until the total numbers of bacteria became 2×108. After that, it was solidified with liquid nutrient agar (Difco) 13 mL. The 10 mm-sized dried paper disks (Toyo Roshi Kaisha, Utsunomiya, Japan) were placed on the plates, soaked with 100 µL supernatants of LAB, and cultured at 37℃ with 5% CO2 for 12 hr. The clear zones were checked, and their diameters were measured with vernier calipers.
From feces of 50 children, a total of 135 LAB strains were identified. Among the strains, five strains (CAU 6728, 7856, 9567, 9967, and 9896), which had larger colonies and grew faster, were selected and examined for physiologic, biochemical characteristics. They had the general characteristics of LAB in arginine and aesculin hydrolysis, acid formation from L-arabinose, lactose, mannitol, and raffinose (Table 2). The three strains, CAU 9567, 9967, and 9896 belonged to the same species.
From the genomic DNA of identified LAB CAU 6728, 7856, 9567, 9967, and 9896, 16S rDNA were amplified by polymerase chain reaction (PCR). 1,525 bp-sized 16S rDNA were amplified (Fig. 1). 16S rDNA sequences of CAU 6728, 7856, 9567, 9967, and 9896 were analyzed by BLAST of NCBI of U.S.A. The 16S rDNA sequences of these LAB were compared with the 16S rDNA of bacteria of GenBank. Similarity matrices were made, and phylogenetic tree was completed (Fig. 2). The range of similarity of 16S rDNA of these LAB were 92.6-99.8%. CAU 6728 was confirmed as Enterococcus faecium, CAU 7856 was confirmed as Lactobacillus brevis, and CAU 9567, 9967, and 9896 were confirmed as Pediococcus acidilactici.
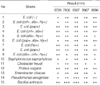
Identified LAB CAU 6728, 7856, 9567, 9967, and 9896 had antimicrobial activities against uropathogenic E. coli and other uropathogens, and they were assessed by checking the diameters of inhibitory zones of LAB at fourth culture day. All five LAB had remarkable antibacterial activities against uropathogens (Fig. 3, Table 3). The most sensitive pathogen to all LAB was Bacillus anthracis, which had two strong positive results. And relatively resistant pathogen was Staphylococcus saprophyticus and Citrobacter freudii, which had three weak positive results.
In an era in which we need new way to treat of urinary tract infection, strategies may include the use of probiotics. Another concept of probiotics is the use of competitive exclusion for improving a specific ecology. Most of probiotic bacteria are comprised of LAB, which has the ability to digest lactose and converting it into lactic acid, so lowering the microenvironmental pH (12). In this group, Lactobacillus, Enterococcus, Streptococcus, Pediococcus, and Bifidobacteria are included.
In this study, we finally got three LAB, Lactobacillus brevis, Enterococcus faecium, Pediococcus acidilactici, and they had profound antibacterial effects in vitro, on the antimicrobial activity assay. These LAB are facultative anaerobic Gram-positive bacteria, vary in their processing of fermentation, hydrogen peroxide and bacteriocin production. They also can be colonized easily in vivo (14). L. brevis, one of these LAB, was the best inhibitor of uropathogens, but others were also good inhibitors (Table 3). It is consistent with previous studies of McGroarty and Reid in adults (13) about Lactobacilli. Enterococcus faecium has good microbiological features such as short generation time and bacteriocin production (15), and also has some success due to their effect in gastrointestinal disorders (16). The three isolated LAB are also popular LAB of intestines in healthy Korean children, so easy to obtain.
In female adults, probiotics have been studied and used for the health of urogenital tract in the area of urogenital infection (17, 18), recurrent superficial bladder cancer (19) and formation of renal stones (20). There is, as yet, no in vivo proof of mechanisms of action in children. In our in vitro study, we selected the candidates for more effective probiotics for in vivo study. We got natural human in vivo probiotics, most well-colonized, with antimicrobial effects.
Some limitations of our study exist. First, the application of these LAB to children has problems to be challenged. There are differences between in vitro circumstance and in vivo circumstance. Of course, the possibility of effects of these three LAB is higher than other LAB, but it cannot be guaranteed. Proposed mechanisms of probiotic LAB in vivo include: 1) adhere to surfaces and inhibit the adhesion of pathogens, 2) inhibit the growth of pathogens, 3) delete nutrients otherwise available to pathogens, and 4) modulate the host immune response and microenvironment, such that risk of infection is reduced (21-23). These are essential conditions for probiotics. On the other hand, for the expected anti-uropathogenic effects, all of the next requisites have to be satisfied. In urogenital tract, the probiotic microflora ascend from rectal skin to urinary tract, kill uropathogens by hydrogen peroxide and bacteriocin-like compounds (13), and reduce iron required by uropathogens but not lactobacilli by iron-withholding system such as siderophilins (24). These require further biochemical study. Second, systemic infection by LAB is possible. In some studies, endocarditis (25), septicemia (26), liver abscess (27) occurred by LAB. And Enterococcus faecium has a fear of the transmission of antimicrobial resistance (28).
Still and all, we can see the possibility of treatment of urinary tract infection by natural human probiotics with least side effects. The three strains are splendid guards in healthy state of the host, and in attack of uropathogenic E. coli and other uropathogens, they would be converted to fighters and inhibit the ascending of the pathogens. We've known the effect of LAB on female adults, but we have to focus the effects of LAB on children, especially in recurrent urinary tract infection, with antibiotics-resistant bacteria, in treatment or prevention. There is also sound rationale for probiotics therapy in urinary tract infection when the LAB of perineal area of male children or vagina of female children are low or absent. We will include in our next study examining the differences of antimicrobial activities of these natural human probiotics and other so-called effective probiotics to urogenital infection in adults (L. rhamnosus GR-1, L. fermentum B-54) (10). The potential of probiotics in this area of pediatric urology is enormous. It is clear that in vivo study is warranted and determination of the role of probiotics in pediatric urinary tract infection is important; whether they are main treatment agents or preventive agents, or just "helpers" or assistants.
Figures and Tables
Fig. 1
Agarose gel electrophoresis of 16S rDNA produced by PCR primed by 27f and 1525r primers from lactic acid bacteria.
Lane M, molecular weight marker; lane 1, CAU 6728; lane 2, CAU 7856; lane 3, CAU 9567; lane 4, CAU 9967; lane 5, CAU 9896.
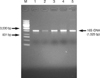
Fig. 2
Phylogenetic tree based on 16S rDNA sequences of lactic acid bacteria and validly described related strains. The tree was constructed by using the neighbor-joining method. Scale bar represent 1 nucleotide substitution per 10 nucleotide.
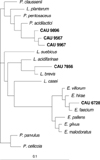
Fig. 3
Inhibitory zone of lactic acid bacteria supernatant against pathogenic bacteria including uropathogenic E. coli (4th day).

References
1. Kunin CE. Urinary tract infections. 1979. 3rd ed. Philadelphia, PA: Lea & Febiger.
2. Newman D. The treatment of cystitis by intravesical infections of lactic Bacillus cultures. Lancet. 1915. 14:330–332.
3. Macfarlane GT, Cummings JH. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ. 1999. 318:999–1003.

4. Floch MH. Buchman A, editor. Prebiotics, probiotics and dietary fiber. Clinical nutrition: a guide for gastroenterologists. 2005. Thorofare, NJ: Slack Incorporated.
5. Tannock GW. Probiotics and Prebiotics: Scientific Aspects. 2005. 1st ed. Norfolk, UK: Caister Academic Press;25–49.
6. Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000. 95:1 Suppl. S11–S13.

7. Guarino A, Canani RB, Spagnuolo MI, Albano F, Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997. 25:516–519.

8. Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000. 30:54–60.

9. Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease : 4-year follow-up of a randomized placebo-controlled trial. Lancet. 2003. 361:1869–1871.
10. Reid G, Bruce AW, Taylor M. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecol Ther. 1995. 23:32–45.
11. Lane S, Evermann J, Loge F, Call DR. Amplicon secondary structure prevents target hybridization to oligonucleotide microarrays. Biosens Bioelectron. 2004. 20:728–735.

12. Mombelli B, Gismondo MR. The use of probiotics in medical practice. Int J Antimicrob Agents. 2000. 16:531–536.

13. McGroarty JA, Reid G. Detection of a Lactobacillus substance which inhibits Escherichia coli. Can J Microbiol. 1988. 34:974–978.
14. Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Envir Microbiol. 1999. 65:351–354.
15. Lewenstein A, Frigerio G, Moroni M. Biological properties of SF68, a new approach for the treatment of diarrhoeal diseases. Curr Ther Res. 1979. 26:967–981.
16. Salminen S, Deighton M. Lactic acid bacteria in the gut in normal and disordered states. Dig Dis. 1992. 10:227–238.

17. Reid G, Bruce AW. Selection of lactobacillus strains for urogenital probiotic applications. J Infect Dis. 2001. 183:Suppl 1. S77–S80.
18. Gardiner GE, Heinemann C, Bruce AW, Beuerman D, Reid G. Persistence of Lactobacillus fermentum RC-14 and L. rhamnosus GR-1 but not L. rhamnosus GG in the human vagina as demonstrated by randomly amplified polymorphic DNA. Clin Diagn Lab Immunol. 2002. 9:92–96.
19. Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, Kitamura T, Kawabe K, Kotake T, Kuroda M, Naito S, Koga H, Saito Y, Nomata K, Kitagawa M, Aso Y. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002. 68:273–280.

20. Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol. 2002. 68:3841–3847.

21. Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties which may influence bacterial interference in the urinary tract. J Urol. 1987. 138:330–335.
22. Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. 2000. 130:2S Suppl. S403–S409.

25. Mackay AD, Taylor MB, Kibbler CC, Hamilton-Miller JM. Lactobacillus endocarditis caused by a probiotic organism. Clin Microbiol Infect. 1999. 5:290–292.

26. Oggioni MR, Pozzi G, Valensin PE, Galieni P, Bigazzi C. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J Clin Microbiol. 1998. 36:325–326.
27. Rautio M, Jousimies-Somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, Koskela M. Liver abscess due to a Lactobacillus rhamnosus strain in distinguishable from L. rhamnosus strain GG. Clin Infect Dis. 1999. 28:1159–1160.
28. Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, Fondén R, Saxelin M, Collins K, Mogensen G, Birkeland SE, Mattila-Sandholm T. Demonstration of safety of probiotics-a review. Int J Food Microbiol. 1998. 44:93–106.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download